Cyanide ion is a chemical compound made up of one Carbon atom and one nitrogen atom with the chemical formula of CN-. These cyanide compounds are poisonous in nature and are often referred to as Cyanide anion or Cyanide ion. This group is also known as the cyano group. This molecule is a conjugate base for Hydrogen Cyanide. Generally, it occurs in the form of white powder or crystals. In its aqueous form, Cyanide smells like bitter almonds.
| Name of molecule | Cyanide Ion ( CN-) |
| No of Valence Electrons in the molecule | 10 |
| Hybridization of CN- | sp hybridization |
| Bond Angles | 180° |
| Molecular Geometry of CN- | Linear |
As mentioned above, this molecule has toxic properties. Hydrogen cyanides formed from this molecule can be harmful to the human body if ingested or inhaled. In this blog post, we are going to look at its Lewis Structure, hybridization and shape.
Contents
CN- valence electrons
To determine the Lewis structure of any given molecule, it is vital to first know the total number of valence electrons for the molecule.
Total number of valence electrons in CN- = Valence electrons of Carbon + Valence electrons of Nitrogen + extra electron ( as this molecule has a negative charge, it has accepted an extra electron which gives it a charge of -1 to this molecule)
Carbon has four valence electrons.
Nitrogen has five valence electrons.
And as there is a negative charge on this molecule, it has an extra electron.
Total number of valence electrons – 4+5+1
= 10 valence electrons
Thus CN- has 10 valence electrons.

CN- Lewis Structure
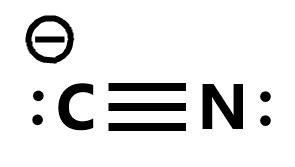
The Lewis structure of any molecule is a pictorial representation of the arrangement of atoms and electrons in the structure along with the bonds formed. The electrons that form bonds with the atoms are called bonding pairs of electrons. The ones that do not take part in bond formation are known as lone pairs of electrons or nonbonding pairs of electrons.
Here as there are two atoms only, place both these atoms next to each other. Now, if you look at the Carbon atom, it requires 4 electrons for completing its octet. Similarly, Nitrogen needs 3 electrons to complete its octet and attain a stable structure.
Carbon and Nitrogen form a single sigma bond first by sharing two electrons. This means that 2 out of 10 valence electrons are used up. But even after forming a single bond, the octets for both these atoms are not complete. Both these atoms form two more pi bonds by sharing four valence electrons.
After forming triple bonds, the octet for Nitrogen is now complete as it has eight electrons in its outer shell. Carbon shares three valence electrons of Nitrogen and an extra valence electron to complete its octet.
As the Carbon atom accepts the extra valence electron, it attains a negative charge. Hence in CN-, there is a formation of triple bonds between Carbon and Nitrogen atoms. The carbon atom accepts an extra valence electron to complete its octet and gets a negative charge of -1. Nitrogen has a lone pair of electrons that doesn’t participate in bond formation as it only shares three out of five valence electrons for forming bonds. And this is the same for Carbon atom as well, given only three out of its four valence electrons participate in forming bonds.
CN- Hybridization
To check the hybridization of the atoms in this molecule, we will use the formula for finding out the number of hybrid orbitals for the atoms. Here Carbon is attached to one atom and has one lone pair of electrons. This means that there is a formation of 2 hybrid orbitals, which means that it has an sp hybridization.
We can also calculate it the other way by using another formula where we find the hybridization by using this formula:
Hybridization of the molecule – 0.5 ( V+M-C+A)
V – Number of valence electrons around the central atom
M – Number of monovalent atoms attached
C – Total cation charge
A – Total anion charge
Hybridization of CN- = 0.5 ( 2+1-0+1)
= 2
The number corresponds to sp hybridization. Thus both atoms, that is, Carbon and Nitrogen, have sp hybridization in CN-. The sp orbitals of both these atoms overlap with each other and form triple bonds.
CN- Molecular Geometry
The molecular geometry of any molecule depends upon the arrangement of atoms and distribution of electrons. In CN-, there are only two atoms forming triple bonds with each other. Both these atoms have a single lone pair of electrons. As the distribution of electrons is quite symmetric for both the sides and there are only two atoms in this molecule, it has a linear molecular geometry.

CN- Bond angle
The bond angles for the CN- is 180 degrees as it has a linear molecular geometry and even distribution of electrons.
CN- Shape
CN- has quite a simple structure to understand. It is a linear molecule, and both the atoms are arranged as far as possible to minimize the repulsive forces of the lone pairs of electrons present on both these atoms. Hence, it has a linear shape.
CN- polar or nonpolar
Cyanide ion is a polar molecule because the bonds formed between Carbon and NItrogen atoms are polar. A carbon atom has an electronegativity of 2.55, and Nitrogen’s electronegativity is 3.04. When the difference of electronegativities is calculated for both these atoms, it is higher than 0.4, making this ion a polar molecule. Hence, cyanide ion has polarity.
Concluding Remarks
To conclude this blog post on Cyanide Lewis structure, we can say that,
- Cyanide molecule has one carbon atom and one nitrogen atom sharing a triple bond.
- There is one lone pair of electrons on both carbon and nitrogen atoms.
- As a Carbon atom accepts an extra electron, it gets a negative charge.
- Both the atoms of Cyanide have sp hybridization and have a bond angle of 180°.
- The overall charge of the molecule is -1 and has a linear molecular geometry.