N2H2 is a chemical formula for a Diazene molecule which is also known as Nitrogen Hydride. It is the conjugate acid of a diazenide. The molecule is made up of two hydrogen atoms and two nitrogen atoms. We will first learn the Lewis structure of this molecule to understand its physical and chemical properties better. The structure will help know the arrangement of atoms, bond formation, and shape of the molecule.
| Name of molecule | Diazene ( N2H2) |
| No of Valence Electrons in the molecule | 12 |
| Hybridization of N2H2 | sp2 hybridization |
| Bond Angles | 109.5 degrees |
| Molecular Geometry of N2H2 | Trigonal Pyramidal |
We will now look at the N2H2 Lewis structure. But for knowing the Lewis structure of any molecule, we first need to know the total number of valence electrons.
Contents
N2H2 Valence electrons
Nitrogen has five valence electrons in its outer shell, but as we already know, there are two nitrogen atoms in this molecule. Therefore we shall multiply the number by 2, which gives us ten valence electrons for both nitrogen molecules.
Hydrogen has one valence electron in its outer shell, but as there are two hydrogen atoms in this molecule, we will multiply the number by 2. So now there are two valence electrons for both hydrogen atoms.
So for the N2H2 molecule, we have a total of 12 valence electrons. (Valence electron on nitrogen + Valence electrons on Oxygen)

N2H2 Lewis structure
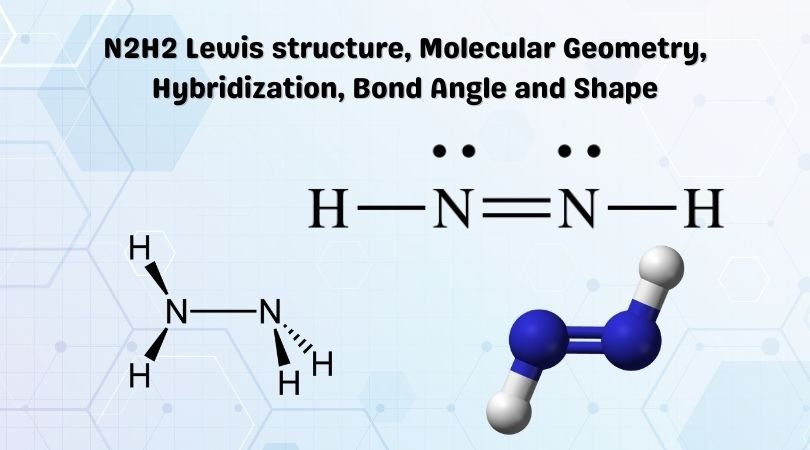
A Lewis structure is a pictorial representation of the arrangement of atoms in a molecule. This is the N2H2 Lewis structure.
Hydrogen atoms always go on the outside, so let’s put the two Nitrogen atoms in the center. After that, we can put an atom of Hydrogen each on the outside of the two nitrogen atoms. So now we’ve used both the Hydrogen atoms and both the Nitrogen atoms as well.
We’ll put a pair of electrons between the Nitrogen and the Hydrogen to form a chemical bond. As Hydrogen needs only two valence electrons for filling the outer shells, we won’t be putting any more there.
Let’s go to the center atoms, the Nitrogen. We’ve used all twelve valence electrons. At this point, one Nitrogen has an octet, but this one Nitrogen right here only has six valence electrons, so that it will need two more.
We can take two valence electrons from the side and move them into the center and form a double bond. To complete the octets on the Nitrogen atoms, we will need to form a double bond between the nitrogen atoms.
We’re only using the twelve total valence electrons we began with, but now both the Nitrogen atoms have their orbitals filled with eight valence electrons. So now all atoms have full outer shells.
N2H2 Molecular Geometry
Nitrogen atoms form a double bond with each other and a single bond with Hydrogen atoms. There are four pairs of bonding electrons and two pairs of lone electrons in this molecule. The lone pairs of electrons are located as far as possible to minimize the repulsive forces between lone pairs of electrons. The molecule takes up a shape to keep the repulsive forces between bonding pairs and nonbonding pairs at a minimum.
Referring to the AXN notation, we can find out the molecular geometry of the molecule.
A is the central atom, X is the number of atoms attached to the central atom, and N is the number of lone electrons’ pairs.
Here when we consider a Nitrogen atom, it is attached to three atoms and has one lone pair of electrons. So the molecule will have AX3N notation, and referring to the table given above, we can say that N2H2 has a trigonal pyramidal molecular geometry.

N2H2 Hybridization
We will find the hybridization for the Nitrogen atoms for this molecule as it takes the central position. By using the formula of Steric Number, we will find out the hybridization for the Nitrogen atoms.
Formula to find Steric Number – Number of atoms attached to the central atom + number of lone pairs on the atom
Each Nitrogen atom is bonded with two atoms and has one lone pair of electrons. Therefore the Steric Number for the Nitrogen atom would be 3, which means it is sp2 hybridized.

N2H2 Bond Angle
The bond angles of the molecules are distorted from the usual for the given molecular geometry due to the presence of lone pairs in the molecule. The bond angles for trigonal pyramidal usually are 109.5°, but here, as there are two lone pairs of electrons, it will be slightly lesser than that.
N2H2 Shape
Diazene or Nitrogen azide has a trigonal pyramidal shape.
Concluding Remarks
To summarize this blog post on N2H2, we can say the following:
- There are 12 valence electrons for this molecule.
- Each Nitrogen atom forms a single bond with one Hydrogen atom and a double bond with the neighboring Nitrogen atom.
- It has four bonding pairs of electrons and two lone pairs of electrons.
- The molecule has a trigonal pyramidal shape with a bond angle less than 109.5°.