The chemical formula ClF5 represents the interhalogen compound Chlorine Pentafluoride. ClF5 is a sweet-smelling, colorless gas at room temperature. The compound is toxic, poisonous, and corrosive. The gas is also a powerful oxidizing agent.
Chlorine Pentafluoride was a well-guarded secret until 1963. Research into rocket propellants was in full swing by the early 60s. Scientists were commissioned to experiment and discover useful chemical compounds to help push past the bounds of space. These experiments resulted in the discovery of trace amounts of Chlorine Pentafluoride, the chemical we’re focusing on today. ClF5 boils at -13.1°C and has a significant density of 1.735 at room temperature.
A report published by D.F. Smith in 1963 illustrates the nature and preparation of the compound. A combination of chlorine trifluoride and fluorine at high temperatures and high fluorine pressures results in a good yield of chlorine Pentafluoride.
ClF3 + F2 → ClF5
ClF + 2F2 → ClF5
Cl2 + 5F2 → 2ClF5
CsClF4 + F2 → CsF + ClF5
Chlorine Pentafluoride reacts vigorously with water to produce Chloryl Fluoride and Hydrogen Fluoride.
ClF5 + 2 H2O → ClO2F + 4 HFClF5 is hypergolic with every known fuel, with no recorded ignition delay. Problems in handling and storage limit its use as a viable oxidizer in propellants. The compound is also a strong fluorinating agent. It is oftentimes incendiary and can cause significant harm if used abhorrently. Vapors released can ignite combustible materials (paper, oil, clothing). The Lewis structure of Chlorine Pentafluoride is especially interesting due to the compounds’ hypervalent state. We will discuss this in the following sections.
ClF5 has the following properties:
| Name of the molecule | Chlorine Pentafluoride (ClF5) |
| No. of valence electrons | 7 + (7 x 5) = 42 valence electrons |
| Hybridization of the central atom | sp3d2 |
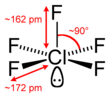
| Bond Angles | 90° |
| Molecular Geometry of ClF5 | Square Pyramidal Molecular Geometry |
Contents
ClF5 Lewis Structure
Lewis dot structures are schematic representations of valence electrons and bonds in a molecule. The simple arrangement uses dots to represent electrons. It gives a brief insight into various molecular properties such as chemical polarity, hybridization, and geometry based on simple models such as the VSEPR and Molecular Orbital theories.
The first step in obtaining a particular Lewis structure is determining the total number of valence electrons available. This will give us a better understanding of structure.
Valence Electrons
Valency can be defined as the combining power of an element. Intuitively, this refers to the number of bonds an element can form during molecular formation. Valence electrons are those electrons that are available for exchanges and bond formation. They are present in the atom’s outermost shell, where the force of attraction from the nucleus is relatively less. This, in turn, makes these electrons readily available for exchange upon excitation.
Before jumping into the Lewis structure, we must determine how many valence electrons are available. Each constituent atom in the molecule contributes valence electrons from their outermost shells. The valence electrons thus calculated are treated as a single unit regardless of their atomic source. Chlorine Pentafluoride comprises two halogenic compounds- Chlorine and Fluorine. Five fluorine atoms are present alongside a lone chlorine atom.
Being in group 7 of the periodic table, chlorine has seven valence electrons with a valency of -1. Chlorine’s electronic configuration is given by [Ne]3s23p5.
Therefore, the lone Chlorine atom contributes 7 x 1 = 7 valence electrons.
Fluorine is in group 17 of the periodic table with the electronic configuration [He] 2s22p5. Therefore, the five Fluorine atoms contribute 7 x 5 = 35 Valence Electrons.
Therefore, the total number of valence electrons in Chlorine Pentafluoride [ClF5] is given by:
7[Cl] + 35[F] = 42 Valence Electrons
ClF5 Lewis Structure Assembly
The first step in ClF5‘s Lewis structure assembly is now complete. We’ve calculated the number of valence electrons to be 42.
Next, a schematic skeletal structure is assembled, depicting the central atom surrounded by the remaining atoms. The central atom acts as a conduit and facilitates bond formation with the surrounding atoms. In Chlorine Pentafluoride’s case, chlorine facilitates the bond formation and takes its place as the central atom. The five fluorine atoms present surround the central chlorine atom, as shown in the figure.

Two valence electrons are placed between atoms to indicate the formed covalent bonds. Ten valence electrons are used up for the five fluorine bonds formed. This can be observed in the figures below.

The red dots represent valence electrons, while the lines between atoms represent covalent bonds. Note that there are ten valence electrons linked to the central chlorine atom. The remaining valence electrons (32) are distributed evenly among the fluorine atoms to fulfill octet requirements.
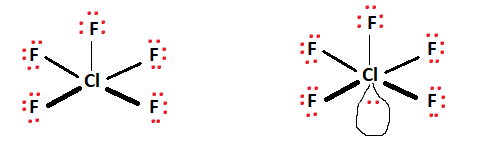
We’re left with a lone pair of electrons that associates itself with the central chlorine atom. All of the valence electrons available have been used up. To verify the Lewis structure’s stability, we can cross-examine each atom against the octet rule. The Fluorine atoms present each have 8 electrons attached (6 non-bonding + 2 bonding, thus fulfilling the octet requirements.
The central Chlorine atom possesses ten bonding electrons (5 covalent bonds) and a lone pair to boot. Therefore, the Chlorine atom possesses more than eight atoms in exception to the octet rule. Why is this so? Chlorine is in the third row (larger size, open d shells) and is capable of hypervalency. Hypervalent elements can possess more than eight electrons, up to 10,12, or even more. Hypervalent compounds are common and no less stable than compounds that conform to the octet rule.
Nevertheless, it pays to be sure. Let’s verify the stability of the Chlorine Pentafluoride Lewis structure using the concept of formal charges.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
Try our Formal Charge Calculator
In this case,
| Element | V | N | B/2 | FC |
| Cl | 7 | 2 | 10/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
| F | 7 | 6 | 2/2 | 0 |
The formal charges being 0 for all of the atoms in the Chlorine Pentafluoride Lewis structure tells us that the structure shown above is stable. Despite the compound being hypervalent, it is stable. Therefore, Lewis Structure for Chlorine Pentafluoride [ClF5] is given below:
![Lewis Structure for Chlorine Pentafluoride [ClF5]](http://geometryofmolecules.com/wp-content/uploads/2022/11/Lewis-Structure-for-Chlorine-Pentafluoride-ClF5.png)
ClF5 Hybridization
Molecular structure and bond formation can be better explained with hybridization in mind. The Hybrid orbitals formed to give a more accurate description of electron regions while also resulting in more stable bonds. Atomic atoms in the central chlorine atom mix to form hybrid orbitals that then bond with the surrounding fluorine atoms.
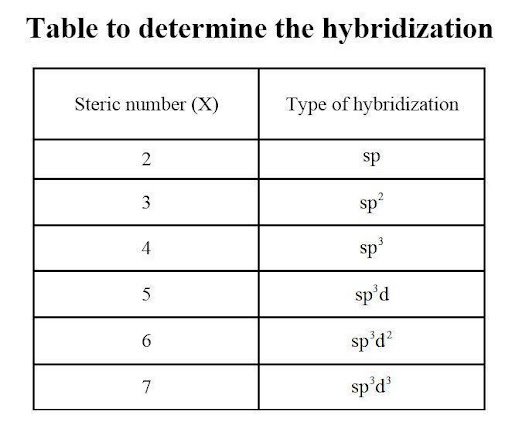
Using the Lewis structure drawn above, we can use the concept of steric numbers to calculate the number of electron domains linked to the central chlorine atom. In this case, there are five covalent bonds and a lone pair of electrons. This gives a hybridization of sp3d2 for the central Chlorine atom. We can also count the number of sigma bonds and lone pairs present to achieve the same result.
ClF5 Bond Angles
Due to the presence of lone pairs, the constituent atoms repel each other and push downwards, per the VSEPR theory. The bond angles in ClF5 are 90°.
ClF5 Molecular Geometry and Shape

To determine the molecular geometry of Chlorine Pentafluoride, we go back to its Lewis structure. Chlorine has a steric number of six (five covalent bonds + one lone pair), meaning that there are 12 valence electrons attached. This is not unusual since chlorine is hypervalent and can possess an expanded octet. Using the steric number chart, we can visualize the addition of atoms to the central atom and observe repulsion in accordance with the VSEPR theory.
The five Fluorine atoms will repel each other while the presence of a lone pair pushes the arrangement downwards to give a square pyramidal molecular geometry.
We can use the A-X-N method to confirm this.
‘A’ here represents the central Chlorine atom. Therefore, ‘A’ = 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, the Chlorine atom is bonded to five surrounding Fluorine atoms.
Therefore, X =5 for the central Chlorine atom.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 1, as a single lone pair of electrons is attached to the central Chlorine atom.
Therefore, that would give us an A-X-N notation of AX5N for the central Chlorine atom in Chlorine Pentafluoride.
From the A-X-N table below, we can determine the molecular geometry for ClF5.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
| AX3N | Trigonal Pyramidal | 109.5 |
| AX5N | Square Pyramidal | 90 |
From the above table, readers can observe that an AX5N arrangement corresponds to a Square Pyramidal geometry. Therefore, Chlorine Pentafluoride has a Square Pyramidal molecular geometry and an Octahedral electronic shape.
Concluding Remarks
Let’s quickly summarize the salient features of Chlorine Pentafluoride
- ClF5 is a sweet-smelling, colorless gas that is extremely dangerous to handle. It is hypergolic with every known fuel and is a very able fluorinating agent.
- ClF5 comprises a central Chlorine atom covalently bonded to five fluorine atoms. There is also a lone pair attached to the chlorine atom.
- Chlorine has 12 valence electrons linked to it. This is not unusual since chlorine is hypervalent and can have an expanded octet.
- The hybridization of the central Chlorine atom in ClF5 is sp3d2.
- ClF5 has a Square Pyramidal molecular geometry and an Octahedral electronic shape with bond angles of 90°.