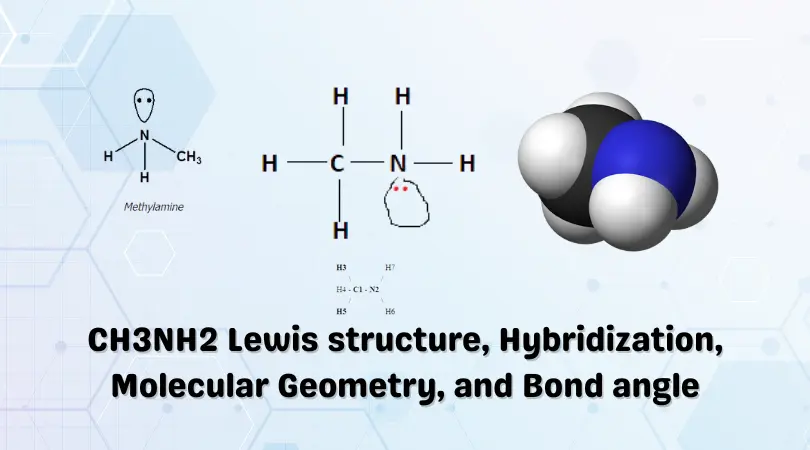
The gas is a derivative of ammonia(NH3) and is heavier than air. CH3NH2 is a formula that represents the organic chemical compound Methylamine. It is a colorless gas with a fishy, ammonia-like odor.
Methylamine is highly flammable and has a flashpoint of -10°C, making precautions necessary during transport and use. It is mixed with portions of methanol, ethanol, oxolane, and water to be sold as a solution. Anhydrous Methylamine gas is also transported in pressurized metal containers.

Methylamines are produced commercially by aminating methanol in the presence of aluminosilicate catalysts (Zeolites). Ammonia reacts with methanol in the vapor phase to produce separate products, namely- MMA(Monomethylamine), DMA(Dimethylamine) & TMA(Trimethylamine). The reaction process and equation are shown below:
CH3OH + NH3 → CH3NH2 + H2O

Methylamine is also produced by the reaction of formaldehyde with ammonium chloride salt. Both gaseous and liquid methylamines are used as intermediates in the production of pharmaceuticals, pesticides, laboratory reagents, and tanning agents. Agrochemicals are the main drivers of the methylamine industry.
With a primary amine group attached to it, Methylamine acts as a good nucleophile.
Other properties of Methylamine(CH3NH2) are given in the table below:
| Name of the molecule | CH3NH2 (Methylamine) |
| No. of valence electrons | (4 x 1) + (5 x 1) + (1 x 5) = 14 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 109.5° |
| Molecular Geometry of CH3NH2 | Trigonal Pyramidal and Tetrahedral |
Contents
CH3NH2 Lewis Structure
Lewis dot structures are schematic representations of valence electrons and bonds in a molecule. The simple arrangement uses dots to represent electrons and gives a brief insight into various molecular properties such as chemical polarity, hybridization, and geometry.
The first step in obtaining a particular Lewis structure is determining the total number of valence electrons available. This helps us understand how the structure is laid out.
Valence Electrons
Valency can be defined as the combining power of an element. Intuitively, this refers to the number of bonds it can form during molecular formation. On the other hand, valence electrons are those electrons that lie in the outermost shell of the atom. Here, the force of attraction from the nucleus on these electrons is weak. Valence electrons break free to participate in the bond formation or electron exchange. This depends on the atom’s and molecule’s electric nature as a whole.
Each atom in the molecule contributes a set number of valence electrons depending upon their atomic number and position on the periodic table. For better understanding, the valence electrons are grouped to build the Lewis structure.
Methylamine comprises two distinct groups- the Methyl group(CH3) and an Amino group(NH2). They come together to form Methylamine(CH3NH2). Let us determine the number of valence electrons present in this molecule.
Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s22p2. Therefore, the lone Carbon atom contributes 4 x 1 = 4 valence electrons
Nitrogen is in group 5 of the periodic table with the electronic configuration 1s22s22p3. Therefore, the lone Nitrogen atom in Methylamine contributes 5 x 1 = 5 valence electrons.
Hydrogen has an electronic configuration of 1s1. Therefore, the 5 Hydrogen atoms contribute 1 x 5 = 5 valence electrons.
Therefore, the total number of valence electrons present in Methylamine(CH3NH2) is given by:
4[C] + 5[N] + 5[H] = 14 valence electrons
Lewis Structure Assembly
The first step to the Lewis structure of Methylamine is complete. The number of valence electrons present in CH3NH2 is observed to be 14.
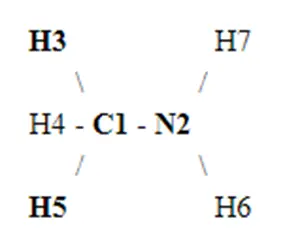
Next, we form a skeletal structure by determining the central atom(s). In this case, both Carbon and Nitrogen are placed adjacent to each other as the central atoms. The Hydrogen atoms present are placed around the two central atoms in accordance with the electronic configuration, i.e., Carbon will have three Hydrogen atoms while Nitrogen will have two. This is illustrated in the figure below:

The valence electrons we calculated earlier are placed between the atoms to indicate covalent bonds formed. Carbon and Nitrogen form bonds with each other and the corresponding Hydrogen atoms. All in all, 12 valence electrons are used during bond formation. This is again shown in the figure below:
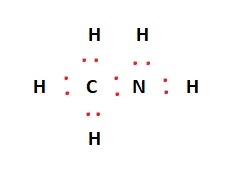
The red dots in the structure represent valence electrons. Two valence electrons are left over. When distributing valence electrons, the aim is to ensure each atom fulfills its outer shell requirements, i.e., adheres to the octet rule. In this case, Nitrogen has six valence electrons. Therefore, the remaining two valence electrons act as a lone pair near the Nitrogen atom in the Lewis structure.

The black lines in the above figure indicate the covalent bond formed due to the sharing of electrons between the atoms. All valence electrons have been used, and the structural arrangement is now stable. The final Lewis structure is given below:

CH3NH2 Hybridization
Molecular structure and bond formation can be better explained with hybridization in mind. The Hybrid orbitals formed give a more accurate description of electron regions while also resulting in more stable bonds. An easy way to determine the hybridization of an atom is to calculate the number of electron domains present near it. The bond between atoms (covalent bonds) and Lone pairs count as electron domains.
Amines contain an Amino(NH2) group with an sp3 hybridized Nitrogen atom bonded to n number of Carbon atoms. In this case, there is a single Carbon atom attached. Carbon and Nitrogen are present as the central atoms in CH3NH2.
Carbon forms sigma bonds with Nitrogen and three surrounding Hydrogen atoms. This gives the Carbon atom an sp3 hybridization state.
Nitrogen forms three sigma bonds with the surrounding atoms. However, the presence of a lone pair leads to another sigma bond, giving the central Nitrogen atom an sp3 hybridization state as well.
Therefore, the Carbon and Nitrogen atoms at the center of Methylamine have sp3 hybridization.
CH3NH2 Bond Angles
According to the VSEPR theory (Valence Shell Electron Pair Repulsion Theory), the lone pair on the Nitrogen and the electron regions on the Hydrogen atoms will repel each other resulting in bond angles of 108.9° near the Nitrogen atom. The Carbon atom will form a tetrahedral shape and result in bond angles of 109.5°.
CH3NH2 Molecular Geometry and Shape
The Lewis structure of a compound gives insight into its molecular geometry and shape. From the Lewis structure, it can be observed that there are two distinct groups of atoms present in Methylamine, i.e., a Methyl(CH3) group and an Amino(NH2) group. Therefore, the molecule will have two distinct geometries on each side.
According to the VSEPR theory, electron regions on atoms will repel each other as much as possible. This repulsion pushed the atoms apart to give molecular geometry. On the Methyl side, four atoms are bonded to the Carbon atom. Upon visualization, you can see that a Tetrahedral shape is formed with Carbon at the center. In contrast, the presence of a lone pair on Nitrogen gives the other end of the Methylamine molecule a Trigonal pyramidal shape.
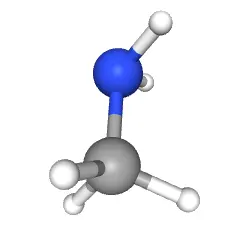

The series of figures above represent a 3-D molecular perspective, with the blue sphere showing the Nitrogen atom bonded to the grey Carbon atom.
We can use the A-X-N method to confirm this. Two groups are present. Therefore, two iterations of the method will be used to determine the geometry.
‘A’ here represents the central Nitrogen atom and Carbon atoms. Therefore, ‘A’ = 1 for both iterations.
‘X’ represents the number of atoms bonded to the central atom. In this case, the Nitrogen atom and two hydrogen atoms are bonded to the central nitrogen atom.
Therefore, X =3 for the Nitrogen atom.
In the case of Carbon, there are three hydrogen atoms and one Nitrogen atom attached to it. Therefore, X = 4 for the Carbon atom. Keep in mind that we’re focusing on two groups within the same molecule.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 1, and a single lone pair of electrons is attached to the central nitrogen atom.
N = 0 for Carbon as no lone pairs are attached to the Carbon atom.
Therefore, that would give us an A-X-N notation of AX3N for the Nitrogen atom and AX4 for the Carbon atom.
From the A-X-N table below, we can determine the molecular geometry for CH3NH2.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
| AX3N | Trigonal Pyramidal | 109.5 |
From the above table, it can be observed that an AX3N arrangement corresponds to a Trigonal Pyramidal geometry. Therefore, the Nitrogen end of the methylamine molecule will have a Trigonal Pyramidal geometry. AX4 corresponds to a Tetrahedral geometry. Therefore, the Carbon end of the Methylamine molecule will have a tetrahedral geometry. The 4th atom in the tetrahedron will be the Amino group.

CONCLUDING REMARKS
Let’s quickly summarize the salient features of Methylamine
- Methylamine is a commercially viable molecule and is produced in large quantities annually.
- The CH3NH2 is organic and comprises a methyl(CH3) and amino(NH2) group.
- Single bonds are formed between the Nitrogen, Carbon, and surrounding Hydrogen atoms. There is also a lone pair attached to the Nitrogen atom.
- The hybridization of the central Nitrogen and Carbon atoms in Methylamine is sp3.
- Methylamine has a trigonal pyramidal molecular structure on the Nitrogen end and a tetrahedral structure on the Carbon end. This results in bond angles of approximately 109.5°.