The chemical formula CH2F2 represents Difluoromethane. Being a refrigerant, Difluoromethane is also known as HFC-32 or, more commonly, R-32. Here, two Fluorine atoms take the place of Hydrogen atoms in Methane (CH4) to form CH2F2. It is a colorless and odorless gas that is also mildly inflammable. It also has exceptional thermal stability and is generally insoluble in water.
CH2F2 is industrially manufactured via the fluorination of Dichloromethane (CH2Cl2). Dichloromethane reacts with Hydrogen Fluoride (HF) in three phases under different conditions.
Owing to its thermodynamic properties, Difluoromethane is used as a refrigerant. CFCs and other aerosols have been ruled as damaging to the ozone layer. As such, hydrofluorocarbons or HFCs are preferred. Difluoromethane has found wide use in Asia and other developing regions. It has an ODP (Ozone Damaging Potential) of 0 and is 675 times more likely to cause Global Warming than Carbon Dioxide. This is still relatively less than other refrigerants like R410A or R417A.
Alternatives to HFCs are being tested, and the United States has begun phasing out the use of Difluoromethane over 15 years.
The Lewis structure of Difluoromethane will help us understand its structure and atomic constitution.
Let us look at some of the properties of Difluoromethane below:
| Name of the molecule | Difluoromethane (CH2F2) |
| No. of valence electrons | 4 + (1 x 2) + (7 x 2) = 20 valence electrons |
| Hybridization of the central atom | sp3 |
| Bond Angles | 109.5° |
| Molecular Geometry of CH2F2 | Tetrahedral Molecular Geometry |
Contents
CH2F2 Valence Electrons
To assemble the Lewis structure of Difluoromethane, we must first calculate the number of valence electrons available. Each constituent atom in Difluoromethane contributes a set amount of valence electrons. This depends on each element’s position in the periodic table and, therefore, its electronic configuration.
Valence electrons, as we know, are electrons that are in the outermost shells of atoms. They can break to take part in bond formation and electron exchanges.
Let’s take a look at how many valence electrons are available to us to assemble the CH2F2 Lewis structure:
First, we have Carbon. Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s22p2. Therefore, a single carbon atom contributes: 4 x 1 = 4 Valence Electrons.
Next, we take a look at Hydrogen. Hydrogen has an electronic configuration of 1s1. Therefore, the two Hydrogen atoms contribute 1 x 2 = 2 valence electrons.
Finally, we have two Fluorine atoms. Fluorine is in group 17 of the periodic table with the electronic configuration [He] 2s22p5. Therefore, the two Fluorine atoms present contribute: 7 x 2 = 14 Valence Electrons.
Therefore, the total number of valence electrons in CF4 is given by:
4[C] + 2[H] + 14[F] = 20 Valence Electrons
CH2F2 Lewis Structure
The Lewis structure represents the assembly of individual atoms and electrons within that compound. It also shows the chemical bonds present and gives much-needed insight into molecular geometry and chemical polarity, among other things.
We know the number of valence electrons available for placement from the previous section. Now, we can assemble the Lewis structure for CH2F2.
Carbon as the central atom here makes sense. With four valence electrons, it can facilitate chemical bonding with the four other atoms present. The arrangement would look something like this:
Next, chemical bonds are formed. Two valence electrons are placed in between atoms to form covalent bonds. This is shown in the figure below:
It can be observed that there are eight dots on the structure so far. This means that eight valence electrons have been used. Where do the remaining valence electrons go? Do they go on the Hydrogen atoms? Well, to determine this observe the figure.
The octet and duplet requirements for the Carbon and Hydrogen atoms have been fulfilled. This means that the remaining valence electrons will now be placed on the Fluorine atoms instead.
Therefore, from the above figure, it is observed that all 20 valence electrons have been placed systematically. We also observe that the octet/duplet requirements for all the atoms in CH2F2 have been met. This means that the Lewis structure assembled is stable and can be further examined to understand the various properties of Difluoromethane.
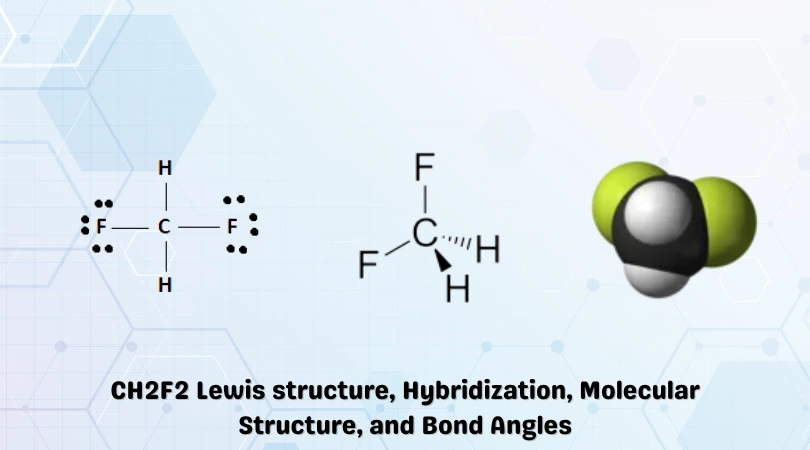
The final Lewis structure is given below:
CH2F2 Hybridization
Hybridization of the Carbon atom in Difluoromethane can be predicted in several ways. We observe that there are four covalent bonds present in the molecule. This gives us a steric number of 4 and, as such, results in a hybridization of sp3 for the central carbon atom.
A more thorough approach with the VBT (Valence Bond Theory) gives us a more comprehensive look at this process. The electronic configuration of Carbon is [He] 2s22p2. In an excited state, this configuration accommodates three 2p orbitals – [He] 2s12p3.
These orbitals now combine to form hybrid sp3 orbitals that form bonds with fluorine and hydrogen atoms. This has implications on both the bond length and bond angle characteristics of the molecule (Bent’s rule)
The more electronegative Fluorine atoms will bond to the p-facing orbital, increasing bond length.
Therefore, it can be said that the hybridization of the central carbon atom in Difluoromethane is sp3.
CH2F2 Bond Angles
The Hydrogen and Fluorine atoms repel each other in accordance with the VSEPR theory, giving the CH2F2 molecule a tetrahedral shape with bond angles of 109.5°.
CH2F2 Molecular Geometry
Molecular geometry is one of the properties that can be ascertained by taking a glance at the Lewis structure obtained. It can be noted that four atoms surround the central Carbon atom. With Carbon at the apex and the other atoms repelling each other, the molecule takes up a tetrahedral shape.
This can be verified using the A-X-N method.
‘A’ represents the central atom which is Carbon in this case. Therefore, A = 1.
‘X’ represents the number of atoms bonded to the central atom. There are four atoms bonded to the central Carbon atom in this case.
Therefore, X =4.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 0.
Therefore, that would give us AX4 for the CH2F2 molecule. Now, using the help of the table given below, we can determine the molecular geometry of Difluoromethane.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
We observe that an AX4 formula corresponds to a molecular geometry that is Tetrahedral. Therefore, CH2F2 has a tetrahedral molecular shape.
Concluding Remarks
Let’s quickly summarize the salient features of Carbon Tetrafluoride
- CH2F2 or Difluoromethane is an organic compound that is also called R-32.
- Due to its exceptional efficiency and thermodynamic stability, it is being widely used as a refrigerant.
- Two fluorine atoms are substituted instead of two hydrogen atoms in CH4 to give Difluoromethane.
- Carbon forms covalent bonds with the surrounding atoms.
- The hybridization of the CH2F2 is sp3.
CH2F2 has a Tetrahedral molecular structure and shape with bond angles of 109.5°.