The chemical formula SiS2 represents the compound Silicon Sulfide. The substance is an inorganic solid with an odor similar to rotten eggs. It is solid at room temperature and can be harmful when not handled with care.
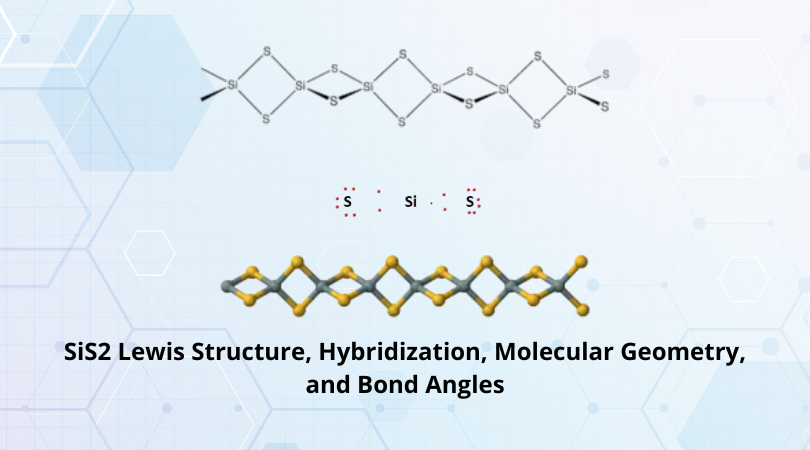
Silicon Sulfide is polymeric and forms tetrahedral SiS4 chains. It can be observed from the figure that Si is bonded to 4 Sulfur atoms.
The compound is formed by an exchange reaction comprising SiO2 and Al2S3. Corresponding to its Sulfide nature, it hydrolyzes readily to release H2S. Reactions with other metallic sulfides result in thiosilicates being formed.
Silicon and Sulfur based compounds are being looked at as potential energy storage alternatives to Graphite and other carbon-based anodes. A recent breakthrough in battery technology suggests that Silicon stabilizes as an anode in the presence of a solid sulfur-based electrolyte. This opens up room for future research and many potential applications.
Some of the salient properties of SiS2 are given below:
| Name of the compound | Silicon Sulfide (SiS2) |
| No. of valence electrons | 4 + (6 x 2) = 16 valence electrons |
| Hybridization of the central atom | sp |
| Bond Angles | 180° |
| Molecular Geometry of SiS2 | Linear Molecular Geometry |
Contents
SiS2 Valence Electrons
Valence electrons are the building blocks of a Lewis structure. They represent the electrons being exchanged and the covalent bonds being formed.
Valence electrons are those that are found in the outermost shell of the atom. They can break away easily to participate in the formation of molecules or compounds due to the relatively weak nature of their attraction/bond to the corresponding nucleus.
Each atom in the compound contributes a set amount of valence electrons. There are two Sulfur atoms and one Silicon atom in the SiS2 molecule, and their contributions in terms of valence electrons are as follows:
Silicon is in group 4 of the periodic table and has an electronic configuration of [Ne] 3s²3p². Therefore, the lone Silicon atom contributes 4 x 1 = 4 valence electrons Sulfur is in group 6(Chalcogens) of the periodic table with the electronic configuration [Ne] 3s²3p4 Therefore, the two Sulfur atoms present in SiS2 contribute 6 x 2 = 6 valence electrons
Thus, the total number of valence electrons available to form Silicon Sulfide [SiS2] is given by:
4[Si] + 12[S] = 16 valence electrons.
With the valence electrons in hand, we can now build up the Lewis structure for Silicon Sulfide.
SiS2 Lewis Structure
The Lewis structure of a compound or molecule represents a schematic arrangement of its components, i.e., its atoms, bonds, and electrons. This, in return, gives us insight into properties such as its molecular structure, polarity, and bond nature.
Lewis structures are tools that can be incredibly useful at only a glance. They are easy to build and provide us with a fair share of data about the particular molecule.
The first step to obtaining the Lewis structure is to calculate the number of valence electrons available. As calculated in the previous section, we have 16 valence electrons available for placement.
Next, we arrange the atoms coherently to facilitate bond formation. Silicon acts as the central atom here, and two Sulfur atoms are placed adjacent to it.
Electrons are placed between the atoms to form covalent bonds. This is shown in the figure below:


We now begin fulfilling the octet requirements for the atoms in the SiS2 molecule. The empty outermost shells are filled first for the Sulfur atoms and then for the Silicon atom. This process can be better understood with the help of the figure below.
It can be observed that both the Sulfur atoms have had their octets filled with valence electrons. However, the central Silicon atom only has four valence electrons attached, leaving its octet incomplete.
We can address this by forming covalent bonds between the Silicon and Sulfur atoms. Electrons are transferred to form these bonds. This process is represented in the figure below:
We observe from the above figure that the central Silicon atom has eight atoms attached to it. Therefore, the octet requirement for all of the constituent atoms in the SiS2 molecule has been fulfilled. This is a key step in the formation of the Lewis structure.
Therefore, the final Lewis structure for SiS2 is as follows:
SiS2 Hybridization
The hybridization of a compound or molecule helps determine the result and nature of new orbital formation when a compound is formed. These new orbitals are of equal energy and can be represented diagrammatically.
An easy way to determine the hybridization of a molecule is to calculate its steric number. The steric number represents the number of covalent bonds or lone pairs present in a particular molecule. A steric number of 2, 3, or 4 corresponds to a hybridization state of sp, sp2, and sp3, respectively. Any further additions go into the ‘d’ orbital.
In the case of Silicon Sulfide, there are two double bonds which essentially translates to there being two covalent bonds in the molecule. This means that it has a steric number of 2.
Therefore, the corresponding hybridization of SiS2 will be sp.
SiS2 Bond Angles
According to the VSEPR theory, the Sulfur atoms repel each other forming a linear molecular shape. As such, the bond angle of SiS2 is 180°.
SiS2 Molecular Geometry
As discussed above, the Lewis structure of a molecule gives us insight into its molecular geometry. From the Lewis structure of SiS2, we observe that Silicon acts as the central atom and that there are two Sulfur atoms bonded to it on either side.
The Sulfur atoms repel each other in accordance with the VSEPR theory resulting in a Linear shape with bond angles of 180°.
We can also verify this using the A-X-N method.
‘A’ represents the central atom, which is silicon (Si). The value of ‘A’ here is 1.
‘X’ represents the number of atoms bonded to the central atom. There are two Sulfur atoms bonded to the silicon atom in this case.
Therefore, X =2.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 0 as no lone pairs are present. The constituent atoms in the SiS2 molecule have attained a complete octet.
Therefore, that would give us AX2 for the SiS2 molecule. From the A-X-N table below, we can determine the molecular geometry.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
The AX2 formula corresponds to a linear molecular geometry. Some of you reading this may have visualized or predicted the same using the Lewis structure above.
The crystalline structure depicted earlier in the section shows an edge-sharing tetrahedral structure where each Silicon atom is bonded to 4 Sulfur atoms.
Concluding Remarks
Let’s quickly summarize the salient features of Silicon Sulfide (SiS2)
- SiS2 is an inorganic solid at room temperature. It has a pungent odor similar to that of rotten eggs.
- SiS2 is being researched for its potential as an energy storage solution.
- Silicon is the central atom and facilitates the formation of double bonds with the adjacent sulfur atoms.
- The hybridization of the SiS2 is given by sp.
- SiS2 has a linear molecular structure with bond angles of 180°.