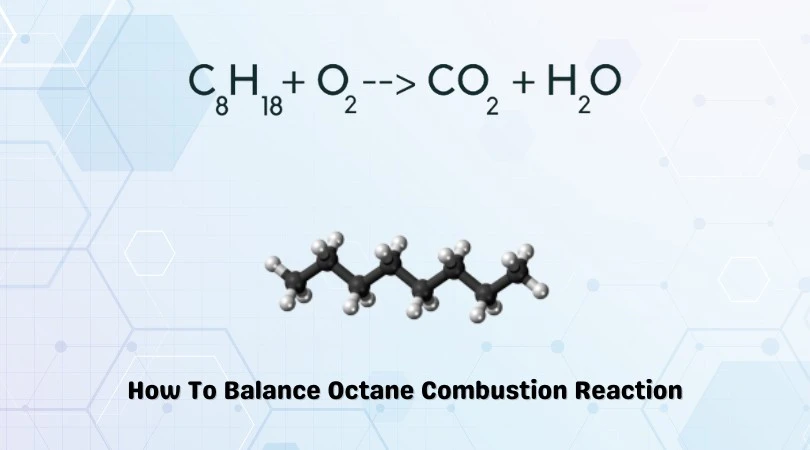
C8H18 + O2 = CO2 + H2O
The above equation represents the combustion of Octane. Octane has a chemical formula of C8H18. It is a volatile and heavily flammable compound. It is also a component of ‘gasoline.’
To balance the equation mentioned above, we must split the equation into ‘reactant’ and ‘product’ halves. The left-hand side represents the reactants involved, and the right-hand side of the equation represents the products.
Once this has been established, we must then calculate the number of atoms present of the corresponding atoms on both sides of the equation.
There are three compounds present in the equation. Carbon is denoted by ‘C,’ Hydrogen represented by ‘H,’ and, Oxygen indicated by ‘O’.
Let us draw two tables to represent the number of atoms on each side.
| Reactants | |
| Carbon (C) | 8 |
| Hydrogen (H) | 18 |
| Oxygen (O) | 2 |
| Products | |
| Carbon (C) | 1 |
| Hydrogen (H) | 2 |
| Oxygen (O) | 3 |
Now, from the table above, we can see that there is a difference between the number of atoms present.
We can modify the equation as follows to balance it. Let us take care of the Carbon and Hydrogen atoms first. Let us use whole numbers to multiply the atoms on the product side.
C8H18 + O2 = 8CO2 + 9H2O
Let us now take a look at the modified table.
| Reactants | |
| Carbon (C) | 8 |
| Hydrogen (H) | 18 |
| Oxygen (O) | 2 |
| Products | |
| Carbon (C) | 8×1 = 8 |
| Hydrogen (H) | 9×2 = 18 |
| Oxygen (O) | 16+9 = 25 |
The Carbon and Hydrogen atoms have been balanced. However, there are 25 Oxygen atoms on the product side. We can multiply the Oxygen atoms on the LHS by 12.5 to get 25 Oxygen atoms, but it is always better to use whole numbers.
Thus, we now multiply the whole equation by 2 to get the following:
2C8H18 + 25O2 = 16CO2 + 18H2O
Let us now take a look at the table below to verify if the above equation has been balanced or not:
| Reactants | |
| Carbon (C) | 8×2 = 16 |
| Hydrogen (H) | 18×2 = 36 |
| Oxygen (O) | 25×2 = 50 |
| Products | |
| Carbon (C) | 16×1 = 8 |
| Hydrogen (H) | 18×2 = 36 |
| Oxygen (O) | 32+18 = 50 |
Therefore, from the above table, we can observe that the number of electrons on either side of the equation equals. This means that the chemical equation is balanced.