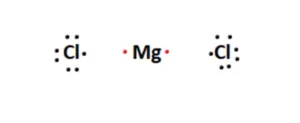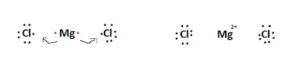The chemical formula MgCl2 represents Magnesium Chloride. The compound is an ionic halide making it highly soluble in water. Hydrates of Magnesium Chloride MgCl2(H2O)x exist abundantly in lakes, seabeds, and seawater.
The compound dissociates into a cation and anion, with Mg2+ being the cation and two Cl- anions. This reaction is shown below:
MgCl2 Mg2+ + 2Cl-
Being hydrates, they lose water at higher temperatures. Anhydrous Magnesium Chloride is obtained industrially by heating a chloride salt of [Mg(NH3)6]2+.
Anhydrous MgCl2 is predominantly used as a precursor to obtaining Magnesium metal. Mg2+ is reduced to Mg0 by electrolysis in the presence of molten salt. Magnesium is produced at the cathode while the chlorine ions are oxidized and released at the other end.
A well-known application of MgCl2 is in the de-icing of highways and yards. In this case, Magnesium Chloride prevents the ice from bonding to the roads and pavements, ensuring easy removal.
It is also used as an essential ingredient in preparing soy milk and tofu, among other things.
Magnesium is an essential component in both plants and humans. Therefore, it also finds use in nutrition aids and pharmaceutical products.
Some of the properties of the MgCl2 are given below:
| Name of the ion | Magnesium Chloride (MgCl2) |
| No. of valence electrons | 2 + (2 x 7) = 16 valence electrons |
| Hybridization of the central atom | – |
| Bond Angles | – |
| Molecular Geometry of MgCl2 | Lattice Structures (6- coordinate Octahedral) |
Contents
MgCl2 Valence Electrons
Building a Lewis structure for any given compound requires that the valence electrons present be calculated first. Each constituent element/atom in the structure contributes a set amount of “valence” electrons based on its position in the periodic table of elements.
These valence electrons are present in the outermost shells of these atoms and can break away. This is because the nuclear force binding them to their shells is relatively weak. Therefore, these electrons can break away to lend a role in forming different types of bonds.
In this case, the ionic halide MgCl2 comprises a lone Magnesium atom and two Chlorine atoms. Let’s take a look at how each atom contributes its valence electrons.
Magnesium is an alkali metal found in group 2 of the periodic table. It has an electronic configuration of [Ne]3s2. Therefore, a single Magnesium atom contributes 2 x 1 = 2 valence electrons.
Now, let’s calculate the same for chlorine.
Being in group 7 of the periodic table, chlorine has seven valence electrons with a valency of -1. Chlorine’s electronic configuration is given by [Ne]3s23p5.
Therefore, the two Chlorine atoms contribute 7 x 2 = 14 valence electrons.
Now, the total number of valence electrons available in MgCl2 is given by:
2[Mg] + 14[Cl] = 16 valence electrons.
MgCl2 Lewis Structure
The Lewis structure is a schematic figure that represents molecular arrangement. It also gives insight into the bond angles, structure, and electronic configuration at first glance. Going further, it tells us about chemical polarity and the nature of the chemical bonds present, among other properties.
The Lewis structure is quite a handy tool, and learning how to draw one up from scratch is essential.
First, we must calculate the valence electrons available to us. These valence electrons are the building blocks of the structure alongside the individual atoms. We have calculated the number of valence electrons in the previous section. This number comes up to 16.
Next, we arrange the Magnesium and Chlorine atoms. Magnesium is the central atom, and the Chlorine atoms are placed adjacent to it. This is shown in the figure below:
The next step is to form chemical bonds. Usually, these bonds are covalent, i.e., the two atoms in focus share their electrons. However, the atoms present are from the alkali metal group and the halogen group in this case. Magnesium is a metal, and chlorine is a non-metal. Therefore, the nature of the bond will be ionic. Each Chlorine atom has seven valence electrons attached to it, while the Magnesium atom has two.
As shown in the figure above, the Chlorine atoms need each electron to complete their octets. Instead of sharing the electrons with ChlorineChlorine, the Magnesium valence electrons are ‘transferred’ over onto the Chlorine atoms.
Therefore, the Magnesium atom gains a +2 charge. This arrangement is represented below. Each Chlorine atom gains a -1 charge, having received an electron. This ionic bond is stable and forms the basis for the Lewis structure of Magnesium Chloride.
Brackets are used below to describe the ionic nature of each of the elements.
MgCl2 Hybridization
Hybridization is when orbitals from two atoms mix to form orbitals of equal energies in the resulting molecule. These hybridized orbitals then take part in chemical bonding.
It helps explain the covalent nature of molecules and how this bond is formed. However, in the case of MgCl2, an ionic bond is present. This means that the bond is formed by the complete transfer of electrons from the electropositive element to the electronegative one. Here, Magnesium transfers its electrons over onto the electronegative chlorine atoms.
Ionic bonds are non-directional, and therefore, the orbital energies don’t come into the picture. Electrostatic forces present here will be our main concern.
Therefore, we need to worry about the hybridization of MgCl2.
MgCl2 Molecular Geometry and Bond Angles
To obtain the above properties, i.e., molecular geometry and bond angle, we usually observe the Lewis structure. This structure will tell us about the electronic configuration, and arrangement of atoms- basically giving us some insight into the molecular geometry and shape. We further flesh this out using the VSEPR theory.
Now, the VSEPR theory is applicable to an arrangement where electrons are shared and to polyatomic ions. Polyatomic ions are ions that contain more than one atom. Example: (OH)- – Hydroxide ion.
In this case, we observe that there is an ionic bond present between Magnesium and Chlorine. However, MgCl2 is not polyatomic, and therefore the VSEPR theory or the A-X-N method cannot be used to determine the molecular geometry of MgCl2.
As shown in the figure, MgCl2 forms crystal lattice structures. Coordinate geometry is used to describe the structure and arrangement of the atoms present.
Concluding Remarks
Let’s quickly summarize the salient features of MgCl2
- MgCl2 consists of a lone Magnesium atom surrounded by two Chlorine atoms.
- In its most stable state, Magnesium acts as the central atom and forms an ionic bond with the Chlorine atoms.
- Owing to the nature of the bond being ionic, the hybridization of MgCl2 can be disregarded. Instead, electrostatic forces and polarity are the main focus.
- Since MgCl2 is not a polyatomic ion, we cannot use the VSEPR method to describe its molecular geometry. Therefore, Coordinate geometry is used to describe the crystalline structure formed.