The chemical formula HCOOH represents Formic Acid. The compound is also referred to as a ‘Methanoic Acid’. In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes. It is soluble in water and polar solvents. Formic acid exists in a dimer form in the vapor phase as well as in Hydrocarbons.
Formic acid owes its name to the Latin word ‘Formica,’ which translates to ants. Some ants and other insects use formic acid to ward off predators or other threats.
HCOOH can be obtained via several processes. The reaction of Carbon Monoxide with Methanol in the presence of Sodium Methoxide gives Methyl Formate. This intermediary undergoes hydrolysis to give Formic Acid.
CH3OH + CO → HCO2CH3
HCO2CH3 + H2O → HCOOH + CH3OH
The above reaction requires an excess of water and can be inefficient. Some manufacturers have worked around this by employing novel methods such as liquid-liquid extraction to separate Formic acid from water.
Formic acid is also obtained as a byproduct in the production of acetic acid through oxidation. Oxidation of biomass, electrochemical reduction, and biosynthesis are other methods through which Formic acid can be obtained.
HCOOH is also the simplest carboxylic acid. It is used mainly as a preservative for livestock feed. It is also used as a soldering agent, a tanning agent in the leather industry, and as an alternative to hydrogen in fuel cells.
Formic acid can cause mild irritation and blisters. Long-term exposure can lead to chronic kidney disease. Some of the properties of HCOOH are given below:
| Name of the molecule | HCOOH |
| No. of valence electrons | (2 x 1) + (4 x 1) + (6 x 2) = 18 valence electrons |
| Hybridization of the central atom | sp2 |
| Bond Angles | Approximately 120° (124-126) |
| Molecular Geometry of HCOOH | Trigonal Planar Molecular Geometry |
Contents
HCOOH Valence Electrons
To determine the Lewis structure of a particular compound, we must first determine the number of valence electrons available to us. Valence electrons are present in the atom’s outermost shell and can break away to form chemical bonds.
These are used to represent chemical bonds and octets on the Lewis structure.
Let us now calculate the number of valence electrons available to us in HCOOH.
Carbon is in group 4 of the periodic table with the electronic configuration [He] 2s22p2. Therefore, the single Carbon atom contributes 4 x 1 = 4 valence electrons.
Hydrogen has an electronic configuration of 1s1. Therefore, the two Hydrogen atoms contribute 1 x 2 = 2 valence electrons.
Being in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of -2. Oxygen’s electronic configuration is 1s22s22p4. Therefore, the two Oxygen atoms present in the molecule contribute 6 x 2 = 12 valence electrons.
Therefore, the total number of valence electrons in Formic Acid (HCOOH):
4[C] + 2[H] + 12[O] = 18 Valence Electrons
HCOOH Lewis Structure
The Lewis structure of a molecule gives insight into its various properties. Some of these include molecular structure and polarity. In addition to representing the basic structure of the molecule, it is an essential tool for classification and visualization.
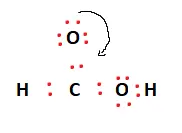
To obtain the Lewis structure of HCOOH, we must first determine the valence electrons available. This has been calculated above to be 18.
Carbon acts as the central atom and facilitates bonding. The other atoms surround it. There are two hydrogen atoms and two oxygen atoms. One of these acts as a hydroxyl (OH) group.
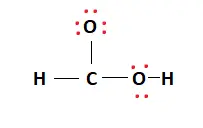
Two valence electrons are placed between atoms to form covalent bonds. This is represented in the figures below.
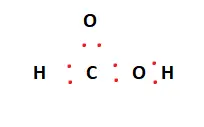
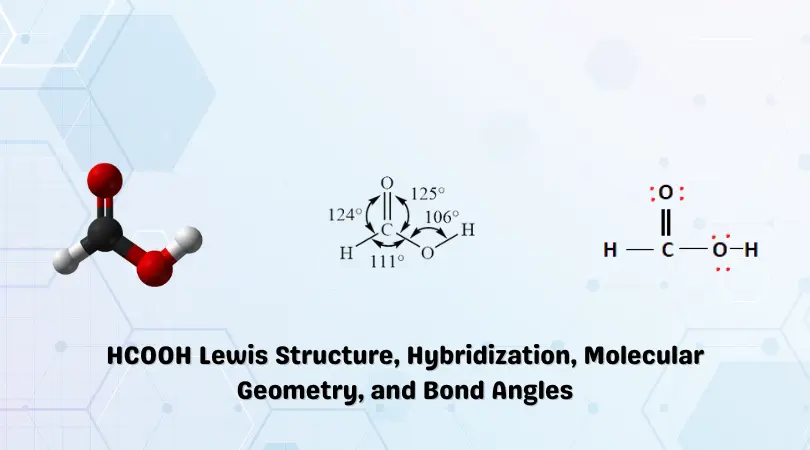
Now that the chemical bonds have been formed, the octets of the constituent atoms must be filled. The remaining valence electrons are then placed around the outermost atoms, consequently filling their outer shells. This is shown below:
The Oxygen atoms now have 8 electrons attached to them. The Hydrogen atoms have also met their outer shell requirements. Carbon has 6 electrons and, as such, needs two more valence electrons to complete its octet.
In this case, we can move two valence electrons from the lone Oxygen atom. This results in a double bond with the central Carbon atom. This is shown below:
In the above structure, it can be observed that all of the constituent atoms in the HCOOH structure have met their outer shell/octet requirements. Carbon now has a double bond with the lone oxygen atom, and they share 4 electrons. This allows both Carbon and Oxygen to meet their octet requirements resulting in a stable structure.
The final Lewis structure for Formic Acid (HCOOH) is given below:
HCOOH Hybridization
The hybridization of the central Carbon atom can be determined by observing the Lewis structure. We can simply count the number of electron domains attached to Carbon to determine the hybridization. Lone pairs and sigma bonds are examples of electron domains.
This is similar to counting the steric number. The steric number represents the number of atoms/lone pairs attached to an atom. Steric numbers of 2, 3, and 4 correspond to an sp, sp2, and sp3, respectively.
In this case, the central Carbon atom is bonded to a Hydrogen atom, an oxygen atom, and a Hydroxyl group (OH). This gives it a steric number of 3.
Therefore, the hybridization of the Carbon atom in HCOOH is sp2.
HCOOH Bond angles
According to the VSEPR theory, the constituent atoms are repelled by each pair resulting in a trigonal planar geometry. As such, the bond angle of HCOOH is theoretically 120°.
However, due to larger repulsion from the double bond, the bond angles are slightly higher. This is represented in the figure below:
HCOOH Molecular Geometry and Shape
To determine the molecular geometry of Formic Acid, we must observe its Lewis structure. The central Carbon atom forms single covalent bonds with a Hydrogen atom and a Hydroxyl group. There is also a double bond with an Oxygen atom.
This gives it a steric number of 3 and suggests a trigonal planar shape. According to the VSEPR theory, the double-bonded oxygen atom repels the other atoms in the molecule.
We can also use the A-X-N concept and its table to verify and determine the molecular geometry of HCOOH.
‘A’ represents the central atom. Carbon is the central atom. The value of ‘A’ here is 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, there are three atoms bonded to the Carbon atom. This can be observed from the Lewis structure above.
Therefore, X =3.
‘N’ represents the number of lone pairs attached to the central atom. There are no lone pairs in the molecule. Therefore, N=0.
Therefore, that would give us a notation of AX3 for the HCOOH molecule. From the A-X-N table below, we can determine the molecular geometry.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
The AX3 formula corresponds to a trigonal planar molecular geometry.
Therefore, Formic Acid (HCOOH) has a trigonal planar molecular geometry.
Concluding Remarks
Let’s quickly summarize the features and properties of Formic Acid (HCOOH)
- HCOOH is also called Methanoic acid. It is the simplest Carboxylic Acid compound.
- Carbon acts as the central atom and forms covalent bonds with hydrogen, Oxygen, and a hydroxyl group.
- The hybridization of the central carbon atom in HCOOH is given by sp2.
- HCOOH has a trigonal planar molecular structure with bond angles of around 120°.