PBr3 is a chemical formula for Phosphorus Tribromide. The molecule is made up of one Phosphorus atom and three Bromine atoms. It is a colourless liquid with a pungent odour. Like PF3 and PCl3, PBr3 also exhibits the properties of both Lewis Acid and Lewis Base.
PBr3 is used in many reactions with alcohol to replace the OH functional group with Bromine atoms. This reaction produces alkyl bromides and is also referred to as a substitution reaction. Phosphorus Tribromide is also used to convert carboxylic acids to acyl bromides. The molecule starts fumes when it comes in contact with moist air due to hydrolysis.
| Name of molecule | Phosphorus Tribromide ( PBr3) |
| No of Valence Electrons in the molecule | 26 |
| Hybridization of PBr3 | sp3 hybridization |
| Bond Angles | 109.5° approximately |
| Molecular Geometry of PBr3 | Trigonal pyramidal |
In this blog post, we will learn about the molecule’s Lewis Structure followed by its molecular shape, polarity and more to understand its chemical properties better.
Contents
PBr3 Valence electrons
To understand the Lewis structure of any given molecule, we first need to know the total number of valence electrons to determine the arrangement of atoms in the structure.
In Phosphorus tribromide, one atom of Phosphorus forms bonds with three Bromine atoms. Hence, we need to find out the valence electrons of both Phosphorus and Bromine atoms.
Total valence electrons in PBr3: Valence electrons of Phosphorus + Valence electrons of Bromine
Phosphorus has five electrons in its outer shell.
Each bromine atom has seven valence electrons, but as there are three atoms of Bromine here, we multiply the number by 3.
Total valence electrons of PBr3 = 5 + 3(7)
= 5 + 21
= 26
There are 26 valence electrons for Phosphorus Tribromide.
PBr3 Lewis Structure
Lewis dot structures or Lewis structures are the diagrams that help to understand the bonding of atoms along with the lone pairs present in the molecule. The valence electrons of atoms form bonds, and these bonds are represented by showing straight lines. Each bond takes up two valence electrons, and the excess electrons are showed as lone pairs.
The electrons that participate in bond formations are called bonding pairs of electrons, whereas the ones that do not take part in forming bonds are termed lone pairs or nonbonding pairs of electrons.
To draw the Lewis structure of an atom, we need to know the following details:
- Total valence electrons of the molecule
- The central atom of the molecule
- Arrangement of other atoms in the molecule
Here we already know that there are 26 valence electrons for PBr3.
And Bromine atom is more electronegative than the Phosphorus atom. Hence the phosphorus atom will take the central position as the least electronegative atom is placed in the centre.
All the bromine atoms will be arranged around the central atom-like shown in the figure below:
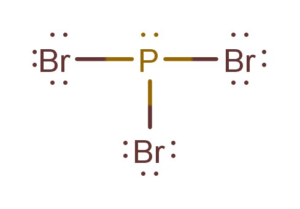
For drawing a Lewis structure, we always first try and complete the octets of the atoms placed around the central atom. The octet rule says that the atoms with eight valence electrons in their outer shell attain a stable structure.
Here we will first try and complete octets of all Bromine atoms, as each Bromine
atom only requires one valence electron to complete its octet.
Draw a line between each bromine and phosphorus atom to show bonds. Each bond here uses up two valence electrons; therefore, a total of 6 valence electrons are used up in forming bonds.
Now, if you notice, forming these bonds will complete the octets of Bromine as well as phosphorus atoms. As both these atoms now have complete octets. Place other valence electrons around all the atoms, and here you have the Lewis Structure of PBr3.
To confirm this structure, one can also calculate the formal charges on the atoms. For this PBr3 Lewis structure, the charges on the central atom are zero, which suggests that this is the right Lewis dot structure for PBr3.
There is one lone pair of electrons on the central atom and three sigma bonds in the PBr3 Lewis Structure.
 PBr3 Hybridization
PBr3 Hybridization
To find out the Hybridization of the given molecule, we always look at the number of hybridized orbitals formed for the bonds formed as well as the lone pairs of the molecule. As mentioned above, there are three bonding pairs and one nonbonding or lone pair of electrons in Phosphorus Tribromide.
The central atom forms four hybridized orbitals to accommodate all these four pairs of electrons. And as there are four hybrid orbitals formed, its Hybridization is sp3. Finding out the steric number or number of hybrid orbitals is one of the most simplified methods to determine the molecule’s Hybridization. One can also use the AXN Notation method to find out Hybridization.
Hence, PBr3 has sp3 Hybridization where there is one s and three p hybrid orbitals formed.
PBr3 Molecular Geometry
The Lewis structure of the molecules helps us understand the arrangement of atoms in the molecule. However, it has some limitations as well. We can’t really depict the molecule’s structure in 3D, and that is where the molecular geometry helps us.
Molecular geometry is the three-dimensional arrangement of atoms in the molecules that aids us to understand the structure of the molecule better. Here we use the VSEPR model to know and understand the molecular geometry of the PBr3 molecule.
As per VSEPR theory, like-charged electrons repel each other, and the molecules take shape to minimize these repulsive forces. For example, bonding pairs of electrons will repel each other, and the same will be the case for lone pairs of electrons.
Here in PBr3, there are three bonding pairs of electrons and one lone pair of electrons. The molecule will adopt a geometry to minimize the repulsive forces. Using the AXN Notation method, we can find out the molecular geometry of the molecule.
A is for the central atom, X is the number of atoms forming bonds with the central atom, and N is the number of lone pairs on the central atom.
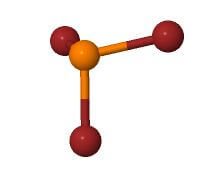
For PBr3, the central atom is Phosphorus, three bromine atoms form bonds with it, and there is one lone pair of electrons. Hence it has AX3N1 notation, and referring to the table below, we can say that it has tetrahedral geometry.
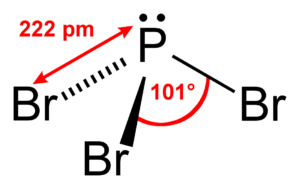
PBr3 Bond Angle
Generally, the molecules with tetrahedral electron geometry and one central atom forming bonds with three atoms have bond angles of 109.5°. However, here, the arrangement of atoms is asymmetrical, and there is a lone pair of electrons on the central atom; the bond angle will not be precisely the same, but it will be around 109.5°.
PBr3 Shape
The above-mentioned AXN Notation method also helps us find the molecular shape, and for PBr3, as it has AX3N1 notation, it has a trigonal pyramidal shape.
PBr3 Polar or Nonpolar
Generally, the molecules with an asymmetric shape are polar in nature due to the uneven distribution of charges. But to confirm it, we will look at the polarity of this molecule.
The electronegativity value of Phosphorus is 2.19 and for Bromine is 2.96. The difference in electronegativities is quite high. As a result, there will be a dipole moment between Phosphorus and Bromine atoms. The direction of this dipole moment will be towards the bromine atom as it is more electronegative, and hence the vector will be placed accordingly.
Along with that, there are three Bromine atoms present in this molecule, which means that dipole moments do not cancel out each other, and as a result, there is a net dipole moment in the molecule. And as there is a dipole moment in this molecule, it is a polar molecule having a partially positively charged region around the Phosphorus atom and partially negative charged regions around Bromine atoms.
Thus, PBr3 or Phosphorus Tribromide is a polar molecule.
Concluding Remarks
To summarize this blog post on PBr3, we can conclude the following:
- Phosphorus Tribromide comprises one Phosphorus atom and three Bromine atoms.
- There are a total of 26 valence electrons for PBr3.
- In the Lewis structure of PBr3, there are three bonding pairs of electrons and one lone pair of electrons on the central atom.
- It has sp3 Hybridization, and the bond angle is approximately 109.5°.
- The molecule is trigonal pyramidal-shaped and is a polar molecule.