Methane is one of the simple organic molecules, given its straightforward structure. It has the chemical formula of CH4 and comprises one carbon atom forming bonds with four hydrogen atoms. The compound is one of the main constituents of natural gas. It is also known as an alkane. Talking about its properties, Methane is a colorless and flammable gas. It is formed by the decaying of natural minerals and is widely used as fuel. CH4 is also used in the natural production of several organic compounds.
| Name of molecule | Methane (CH4 ) |
| No of Valence Electrons in the molecule | 8 |
| Hybridization of CH4 | sp3 hybridization |
| Bond Angles | 109.5 degrees |
| Molecular Geometry of CH4 | Tetrahedral |
In this blog post, we will find the Lewis Structure, Molecular Geometry, and Shape of the molecule. But before proceeding with the Lewis Dot Structure, we will first look at the total number of valence electrons for this molecule as these are the ones that participate in the bond formation.
Contents
CH4 Valence electrons
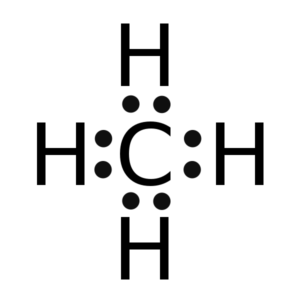
The electrons that participate in the bond formation and are in the outermost shell of the atom are referred to as valence electrons.
Carbon is a p block element present in group 14th and 2nd period in the periodic table and has atomic number of 6. Hydrogen is the very first element in the periodic table having an atomic number 1. So, in Carbon, the electronic configuration will be 1s2 2s2 2p2 and the electronic configuration of Hydrogen will be 1s1. The electrons present in the outermost shell of an atom are termed as valence electrons. This gives us a total of 4 valence electrons in carbon and 1 in Hydrogen. The total number of valence electrons in CH4 adds up to be 8 (4 in carbon and 1 from each hydrogen atom).
Thus there eight valence electrons for Methane.
CH4 Lewis Structure
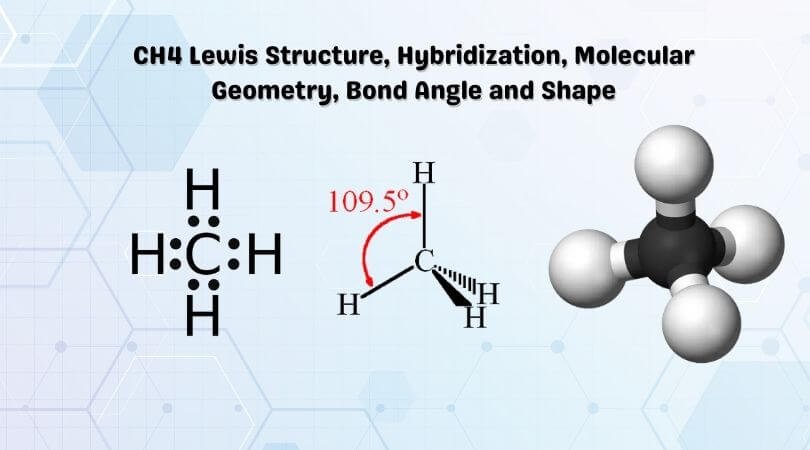
Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms’ bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don’t are known as nonbonding pairs of electrons. Lewis structure is also referred to as electron dot structure.
Dots are represented to show the electrons, and lines are used to indicate the bonds between the atoms. Lewis structures are based on the octet rule, which says an atom must have eight valence electrons in its outer shell to attain a structure similar to the closest noble gas. However, many elements are exceptions to this rule.
Let us look at the Lewis Structure of CH4 and determine how the atoms are arranged in the molecule.
Carbon in Methane takes the central position as it is less electronegative than the Hydrogen atoms. Arrange all the Hydrogen atoms around the Carbon atom.
Now each Hydrogen just needs one more valence electron to attain a stable structure. So for doing that, it will share one valence electron of the Carbon atom.
As Carbon has four valence electrons, it will share all four electrons with the Hydrogen atoms. Each bond requires two valence electrons, and hence eight valence electrons are used up by forming bonds.
So in the Lewis structure of CH4 or Methane, there are four single or covalent bonds between each Hydrogen and Carbon atom. There are four bonding pairs of electrons and no lone pair of electrons in this molecule.
CH4 Hybridization
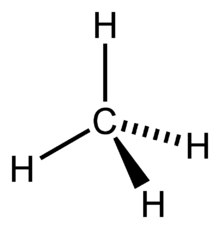
For the CH4 molecule, we will find the Carbon atom’s hybridization as it is the one sharing electrons with Hydrogen atoms and forming bonds. So for finding out the hybridization for the Carbon atom, we will find out the Steric Number.
Steric Number- Number of atoms attached to the central atom + number of lone pairs on the atom
= 4+0
= 4
Hence four hybrid orbitals are formed for CH4, and referring to the table given below, we can say that it has sp3 hybridization. One 2s orbital and three 2p orbitals are hybridized for the Carbon atom.
This is a theoretical concept which states that the atomic orbital of the atoms combines to form completely new molecular orbitals by overlapping.
CH4 Molecular Geometry
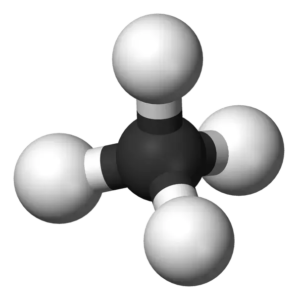
Molecular geometry helps us understand the arrangement of atoms in 3D for any given molecule. For the Methane molecule, there are four covalent bonds between Hydrogen and Carbon atoms. The molecule has quite a symmetry in its arrangement as there are bonds on all four sides of the central atom.
According to VSEPR theory, electron pairs of the same nature repel each other. There are four bonding pairs of electrons, so to keep their repulsive forces at a minimum, they take the tetrahedral molecular geometry.
Hence, CH4 or Methane has a Tetrahedral Molecular geometry.
CH4 Bond Angles
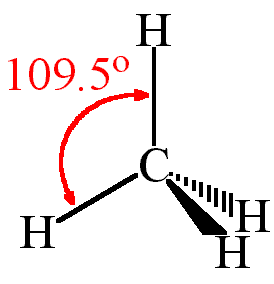
One can use AXN Notation to find out the molecular geometry and the bond angles for any molecule. Here CH4 follows the AX4 notation, and hence according to the table given below, the bond angles are 109.5°
The CH4 molecule will have 109.5° bond angles as there is no distortion in its shape. Generally, the lone pairs in the molecule distort the shape of the molecule, which changes the molecule’s bond angles. But as there are no lone pairs of electrons in this molecule, H-C-H’s bond angle is 109.5°.
CH4 Shape
Methane has a tetrahedral molecular geometry, and thus, it is a tetrahedral shape molecule.
Concluding Remarks
To summarize this blog, we can conclude the following points for the Methane molecule:
- CH4 molecule is made up of one Carbon atom and four hydrogen atoms.
- There are four single bonds in the molecule between Carbon and Hydrogen atoms.
- A total of 8 valence electrons are there for this molecule.
- All the valence electrons are used up in the bond formation; hence the molecule has no lone pairs, and there are four bonding pairs of electrons.
- Carbon has sp3 hybridization, and the molecule takes up a tetrahedral shape to keep the repulsive forces of bonding pairs at a minimum.
- The bond angle of H-C-H is 109.5°.
To know about the polarity of the CH4 molecule, check out our detailed blog post on CH4polarity to find out if the molecule is polar or nonpolar.



The article is very well written.