Beryllium chloride is an inorganic compound having a chemical formula of BeCl2. This compound exists in monomeric solid form as well as forms a polymeric chain. It exists as yellow coloured crystal solids at the standard room temperature. A lot of people get confused when it comes to the polarity of this molecule. In this blog, we will determine the polarity of this molecule.
The molecule’s polarity depends upon certain parameters of the molecule such as the shape of the molecule, net dipole moment, and the distribution of charges in the molecule.
Contents
BeCl2 Shape
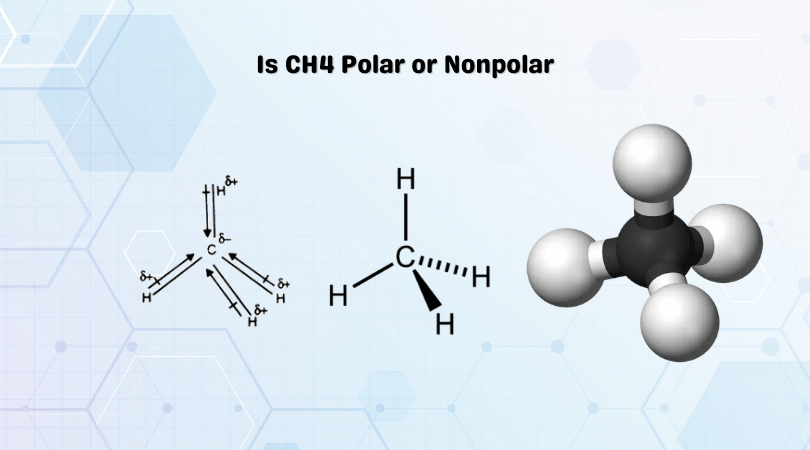
BeCl2 is a linear molecule with a bond angle of 180°. The molecule has no lone pairs, and the bonding pairs of electrons are positioned to avoid the repulsive forces. The molecule has a symmetric distribution of atoms.
BeCl2 net dipole moment
The dipole moment depends upon the difference of electronegativities of the atoms. Beryllium has an electronegativity value of 1.57, and for Chlorine, it is 3.16. The difference in electronegativity is relatively higher than 0.4, which makes Be-Cl bonds polar. Chlorine atom is more electronegative, so it will try to pull the shared electrons towards itself. But as both the Chlorine atoms are placed in opposite directions, their dipole moment will cancel each other. As a result, there is zero or no net dipole moment in BeCl2.
Distribution of charges
The net dipole moment determines the distribution of charges in the molecule. Although there is no net dipole moment, there are uneven charges in the molecule. As the chlorine atom is more electronegative and pulls the electrons towards itself, it has partial negative charges, and the Beryllium atom has partial positive charges.
Is BeCl2 a polar or nonpolar molecule?
BeCl2 or Beryllium Dichloride is a nonpolar molecule as there is zero net dipole moment. The molecule has linear geometry hence the dipole moments in the molecule get nullified. So to conclude, we can say that this molecule is nonpolar.