We have previously discussed the Lewis structures of CO2, O3, SO2, SO3, and more. Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape.
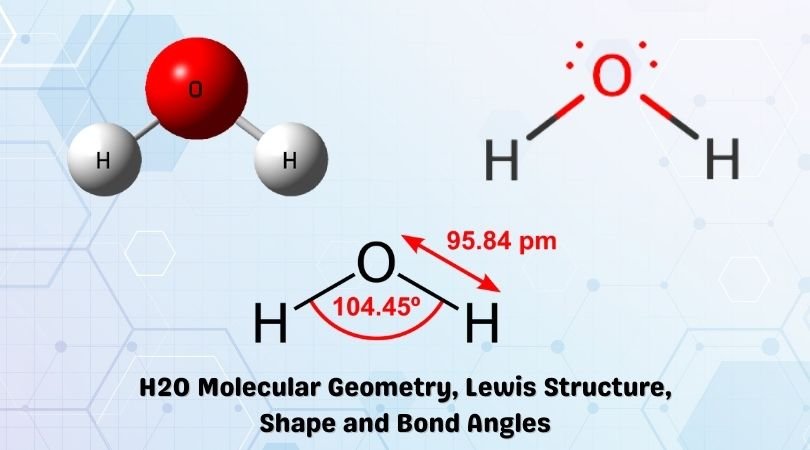
Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. While we always knew chemistry is everywhere, we definitely didn’t know that even water has chemical formulas back in our childhood days. Water has a chemical formula of H2O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide.
| Name of molecule | Water ( H2O) |
| No of Valence Electrons in the molecule | 8 |
| Hybridization of H2O | sp3 hybridization |
| Bond Angles | 104.5 degrees |
| Molecular Geometry of H2O | Bent |
In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles. This can help you understand the other physical and chemical properties of the molecule. But before looking at its Lewis Structure, we will first go through the total number of valence electrons for this molecule as these electrons are the ones that participate in bond formation.
Contents
H2O Valence electrons
To get the total number of valence electrons for this molecule, we will add up Hydrogen and Oxygen atoms’ valence electrons.
Valence electrons of Hydrogen: 1*2 ( as there are 2 Hydrogen atoms, we will multiply it by 2)
Valence electrons of Oxygen: 6
Total number of valence electrons in H2O: 2 + 6
= 8 valence electrons
Thus, H2O has a total of 8 valence electrons.
H2O Lewis Structure
Lewis Structure for any molecule helps to know the bonds formed in the structure and the electrons participating in the bond formation. The electrons that participate in bond formation are known as the bonding pair of electrons. In contrast, the ones that don’t take part in any bond formation are called nonbonding pairs of electrons or lone pairs of electrons.
Here we will first place the atoms and individual valence electrons to understand the Lewis structure of H2O step-by-step.
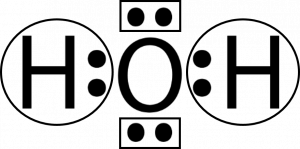
Oxygen atoms will take a central position as Hydrogen atoms always go on the outside. So place Oxygen in the center with both the Hydrogen atoms on the side. Each Hydrogen atom here needs one more valence electron to attain a stable structure. Similarly, an Oxygen atom needs two valence electrons to complete its octet.
Both Hydrogen atoms will share one valence electron of the Oxygen atom to attain a stable structure. For showing the sharing of electrons, show a single bond on both sides.
This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons.
H2O Hybridization
When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. These orbitals help us to predict the hybridization of the molecule. Here we will look at the Oxygen atom’s hybridization as it shares two of its valence electrons with both Hydrogen atoms. Three 2p orbitals of Oxygen and one 2s orbital are hybridized as there are two pairs of bonding electrons and two lone pairs. And as four orbitals of Oxygen are hybridized, the hybridization of H2O is sp3.
H2O Molecular Geometry
The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms, and its electrons. In an H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms.
As the repulsion forces from the lone pairs are more than the repulsive forces of bonded pairs, the arrangement of atoms is distorted. Hence the molecular geometry of the water molecule is angular or v-shaped, and some people also refer to this bond geometry as distorted tetrahedron geometry.
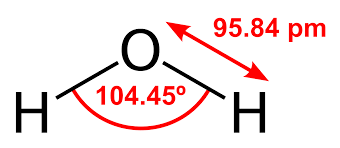
H2O Bond Angle
The bond angle for the molecules having a tetrahedral geometry is 109°, but as the geometry of the H2O molecule is distorted due to the presence of the lone pairs of electrons, the bond angle decreases from 109° to 104.5°
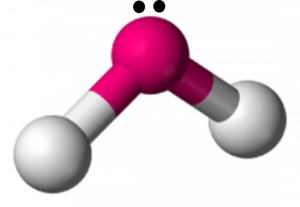
H2O Shape
The molecular shape of the H2O molecule is bent.
Concluding Remarks
- To summarize this article we can say that the H2O molecule comprises two hydrogen atoms and one oxygen atom.
- There are a total of 8 valence electrons for this molecule, out of which four are used to form O-H sigma bonds.
- There are two lone pairs on the Oxygen atom as it doesn’t participate in forming bonds.
- The oxygen atom in the H2O molecule has sp3 hybridization, and the bond angle of H-O-H is 104.5°
- The molecular geometry and the shape of the water molecule are bent due to the repulsion forces of lone pairs.