Phosphorus Pentafluordie is a colourless and toxic gas. It is made up of one Phosphorus atom and five Fluorine atoms. This molecule is also known as the halide gas as it consists of Fluorine (a halogen atom). To understand the physical and chemical properties of this molecule, it is essential to know its Lewis Structure. This structure helps to understand the arrangement of atoms, bond formation and shape of the molecule.
| Name of molecule | Phosphorus Pentafluoride ( PF5) |
| No of Valence Electrons in the molecule | 40 |
| Hybridization of PF5 | sp3d hybridization |
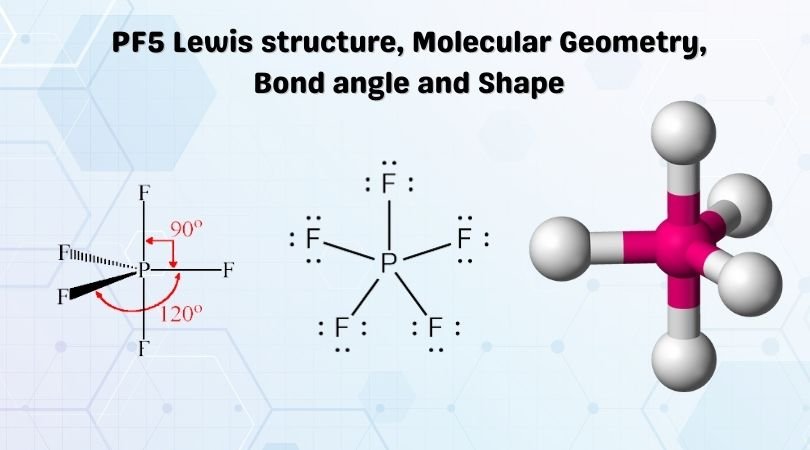
| Bond Angles | 90° and 120° |
| Molecular Geometry of PF5 | Trigonal Bipyramidal |
However, for knowing the Lewis Structure of any compound, one first needs to know the total number of valence electrons. Here in this blog post, we will look at the PF5 Lewis Structure, Hybridization, Bond angle, and shape.
Contents
PF5 Valence electrons
The total number of valence electrons in PF5: Valence electrons of Phosphorus + Valence electrons of Fluorine.
Phosphorus has 5 valence electrons in its outer shell.
Fluorine has 7 valence electrons in its outer shell, but as there are 5 fluorine atoms, we will multiply the number by 5. So we have 35 valence electrons for all Fluorine atoms.
Total number of valence electrons – 5 + 35
= 40
Thus, there are a total of 40 valence electrons for PF5.
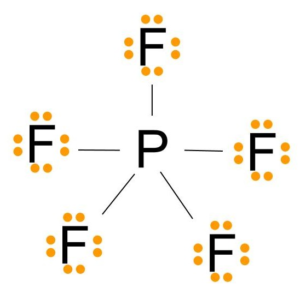
PF5 Lewis Structure
Lewis Structure of a molecule is a pictorial representation of the arrangement of atoms in the molecule. This structure helps us understand the electrons that take part in forming bonds and the arrangement of electrons in the molecule.
Here Phosphorus atom will take the central position as it is less electronegative than the Fluorine atoms. So Phosphorus atoms will be in the center, and all the Fluorine atoms will be arranged around it.
All Fluorine atoms here need one valence electron to complete their octet. Each Fluorine atom will share one valence electron of the Phosphorus atom. Doing so, the octets of all Fluorine atoms will be completed.
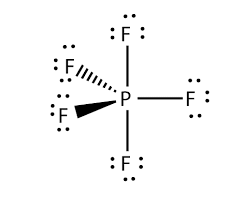
Phosphorus here now has more than eight valence electrons in its outer shell because it has extra hybridized orbitals to accommodate more electrons.
There are no lone pairs in the Lewis Structure of PF5, and there are five single bonds between Phosphorus and Fluorine atoms.
PF5 Hybridization
The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3, but when it is in an excited state, the electrons from 3s orbital get unpaired. There are five half-filled orbitals: one s orbital, three p orbitals, and one d orbital. All these five orbitals accommodate one valence electron of the Fluorine atoms. And as there is the formation of five hybridized orbitals for this molecule, Phosphorus has sp3d hybridization in PF5.
PF5 Molecular Geometry
Phosphorus forms single bonds with all five Fluorine atoms, which means there are five regions of electrons density. All the valence electrons are paired up in this molecule, and as a result, there are no lone pairs of electrons in this molecule. Three Fluorine atoms are located in the equatorial position whereas the other two take the axial position. This arrangement of the atoms makes the molecular geometry of PF5 Trigonal Bipyramidal.

PF5 Bond Angles
As mentioned earlier, the fluorine atoms in PF5 either occupy the equatorial position or axial one; there are two bond angles for this molecule. The bond angles for the Fluorine atoms in the equatorial position, F-P-F is 120°. The angle between the fluorine atoms located in axial and equatorial position is 90°
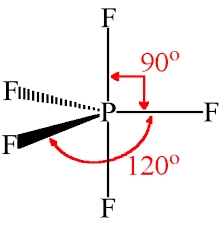
PF5 Shape
Phosphorus Pentafluoride has a trigonal bipyramidal shape as two fluorine atoms occupy the axial position and the other three fluorine atoms are in the equatorial position.
Concluding Remarks
To summarize this blog post on Phosphorus Pentafluoride, we can say that:
- There are five single bonds in this molecule as each Fluorine atom forms a bond with the central Phosphorus atom.
- Phosphorus Pentafluoride has trigonal bipyramidal molecular geometry with sp3d hybridization.
- The bond angles for this molecule are 90° and 120°.