Oxygen Difluoride is one of those gases which was discovered by accident. However, it is not considered safe due to its strong oxidizing properties. The Hydrofluoric acid produced by hydrolyzing OF2 is highly corrosive and toxic. OF2 has an oxidation number of 2, making it a powerful oxidizing agent.
Contents
Polarity of OF2
To determine the polarity of a molecule, we have to consider several factors such as the
- Net dipole moment in the molecule.
- The difference of electronegativities of the atoms in the molecule
- The shape of the molecule.
Here we will go through each of these points one by one.
Electronegativities of the atoms in OF2
In OF2, the Oxygen atom is the least electronegative one and hence it is placed in the centre. Fluorine is highly electronegative and has an electronegative value of 3.98, whereas Oxygen has an electronegative value of 3.44.
The difference between the electronegativities of both these atoms is higher than 0.5, which makes the O-F bonds polar. So both the single bonds formed between Oxygen and Fluorine atoms are polar.
Dipole moment in OF2
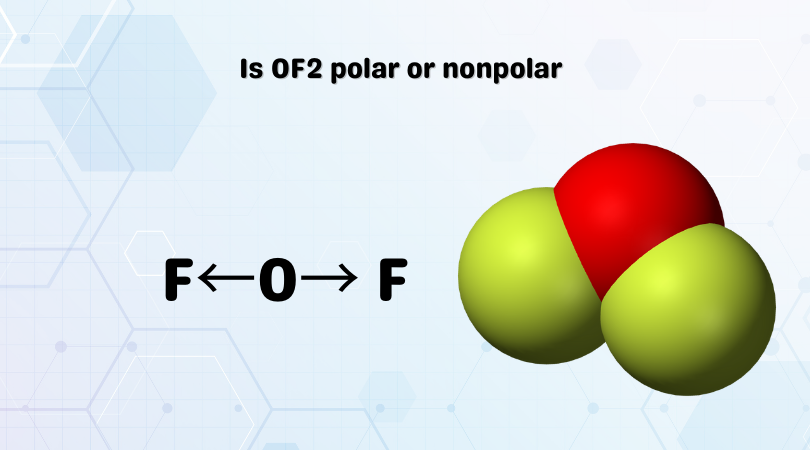
The dipole moment exists in this molecule as the bonds of O-F are polar. This dipole moment will be towards Fluorine from Oxygen, as the Fluorine atom will try to pull the electrons to itself.
As a result, the direction of the dipole moment will be from Oxygen to Fluorine.
F←O→ F
The arrows in the image show the dipole moment.
Shape of OF2
Due to the presence of two lone pairs of electrons on the central Oxygen atom, this molecule’s shape is bent. As the shape is bent now, both these dipole moments are not canceled out. Instead, the dipole moments will sum up, making this molecule a polar molecule.
Concluding Remarks
As the dipole moments are not canceled out in the OF2 molecule, there will be a net dipole moment in it, which means there will be partial negative charges and partial positive charges in the molecule. Thus Oxygen Difluoride or OF2 is a polar molecule.