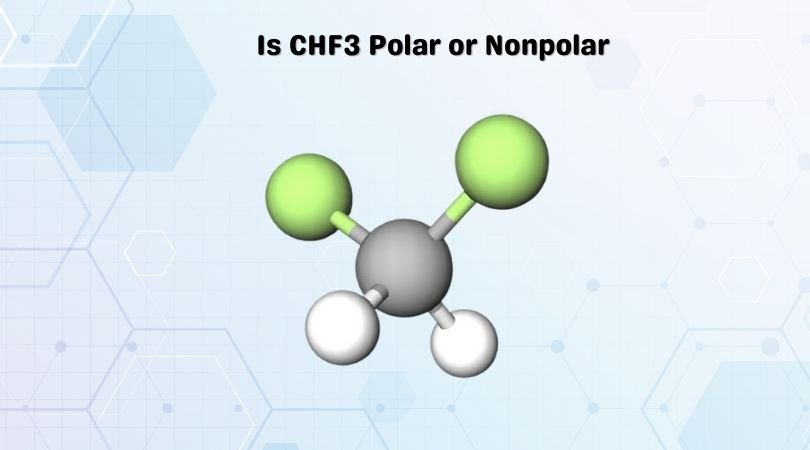
CHF3 is a chemical formula for Fluoroform. It comprises one Carbon atom, three Fluorine atoms, and one Hydrogen atom. To know the polarity of the molecule, it is essential first to understand the Lewis structure, its shape, net dipole moment in the molecule, and the difference in electronegativities of the atoms in the structure.
For Chloroform, this is the Lewis structure.
As one can depict from the structure itself, that it is not a symmetrical structure. Carbon is in the center position forming bonds with three Fluorine and one Hydrogen atom. There are only single bonds formed in this structure, and although it might seem that tetrahedral molecules are nonpolar, we can find out the polarity by considering some factors.
The shape of the molecule

CHF3 has a tetrahedral shape, given the central atom forms single bonds with four atoms at a given time. All fluorine atoms have nonbonding or lone pairs of electrons. Due to such lone pairs, the molecule tries to adapt the shape that can minimize the repulsive forces between all these lone pairs of electrons. Generally, the presence of lone pairs of electrons leads to asymmetry in the structure and distorts the shape a little, causing molecules to be polar.
The difference in electronegativities of atoms
Carbon is the least electronegative atom in this structure. The difference in electronegativities of Carbon and hydrogen is quite less, and hence we do not consider C-H bonds are polar. But when we look at the electronegativity differences for Hydrogen and Fluorine, it is quite high. This is because Fluorine atoms are quite electronegative. . Fluorine has an electronegativity of 3.98, whereas hydrogen has an electronegativity value of 2.2. These differences lead to the dipole moment in the molecule
Net Dipole moment in CHF3
A Hydrogen atom does not have three electron clouds, just like the Fluorine atoms, which depicts that the region near the Hydrogen atom will be more positive than the part where it has three Fluorine atoms. As there is an asymmetry in the molecule due to the electron density regions, there will be a net dipole moment in the molecule and the formation of poles.
Finally CHF3 Polar or Nonpolar?
CHF3 or Fluoroform is a polar molecule, given it is asymmetric and has poles in it. The uneven distribution of charges in the molecule results in the formation of poles for this molecule. Looking at all the characteristics and the net dipole moment in the molecule, we can conclude that this molecule is a polar molecule.