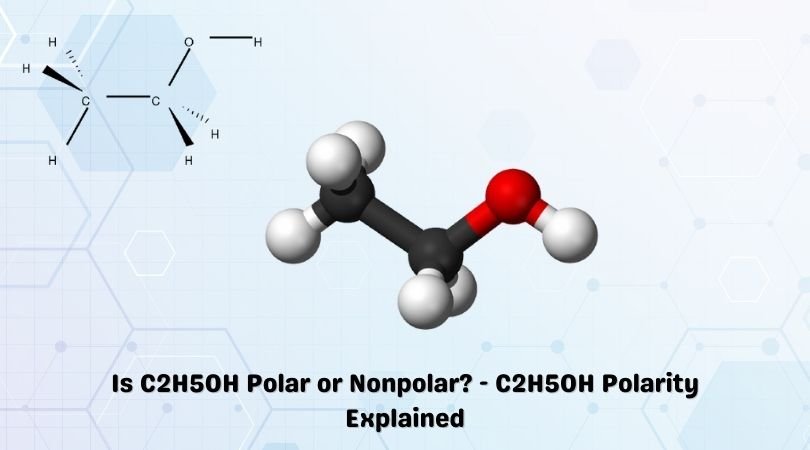
C2H5OH is a chemical formula for Ethanol, an organic chemical compound. It is a flammable, volatile and colourless liquid having a characteristic odour. When it comes to Ethanol and other alcohols, many of our students and readers have doubts regarding their polarity. In this blog post, we will address the questions related to whether Ethanol is polar or nonpolar, along with its uses, properties and a step-wise approach that can help you determine the polarity of any given molecule.
Contents
Is Ethanol polar or nonpolar?
Ethanol is a polar molecule. And we will elaborate on this conclusion and the factors that helped us determine this. But first, you should know the Lewis structure of Ethanol to know the formation of bonds, atoms involved in the structure and other such characteristics.

Electronegativity and Bond Nature in C2H5OH
C2H5OH comprises two Carbon atoms, five Hydrogen atoms and one hydroxyl ( OH) group. When we consider the electronegative values of Carbon, Oxygen and Hydrogen, the Oxygen atom is the most electronegative atom here.
The electronegative values of all the atoms are as follows:
- Carbon: 2.55
- Hydrogen: 2.20
- Oxygen: 3.44
When you calculate the difference of electronegativities for Carbon and Hydrogen, it is less than 0.4, which means that C-H bonds are nonpolar in nature. If you compare the difference of electronegativity for Carbon and Oxygen atoms, it is much higher than 0.4, making the bond between Carbon and Oxygen atoms polar. The O-H bonds are also considered polar due to the higher electronegativity of oxygen atoms.
Net Dipole moment in C2H5OH
As mentioned above, both C-H and O-H bonds are polar, meaning there will be a dipole moment towards the Oxygen atom in both these bonds. The regions around the Oxygen atom will have partial positive charges, and the regions around Carbon and Hydrogen atoms will have partial negative charges.

Given that this molecule is not symmetrical, the dipole moments don’t cancel out each other. As a result, there is a non zero net dipole moment in the C2H5OH molecule, making it a polar molecule.
Physical properties of Ethanol:
- The boiling point of Ethanol ( C2H5OH) is 78.5 °C.
- The melting point of Ethanol is -114.5 °C.
- The molecular weight of Ethanol is 46.06 g/mol.
- It is an excellent solvent and is also known as grain alcohol or alcohol.
- It has a pleasant odour.
Uses of C2H5OH
Ethanol has several uses, including using it as an antiseptic, medical solvent, disinfectants and other such areas. Ethanol is also used as engine fuel and fuel additive. It is a universal solvent as it can dissolve both polar and nonpolar compounds.
Concluding Remarks
To sum it up, we can say that C2H5OH is a polar molecule due to the non-zero net dipole moment in this molecule. As the Oxygen atom is more electronegative than the Carbon and Nitrogen atoms, it leads to dipole moment, which causes the partial distribution of charges in the molecule, making it a polar molecule.