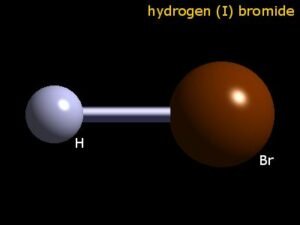
Hydrogen Bromide is an inorganic compound and is also known as a hydrogen halide. It exists in a colourless gaseous form, but when dissolved in water, it forms Hydrobromic acid. Knowing its polarity can help us find out its potential uses and applications.
Hydrogen Bromide or HBr is a diatomic molecule. It comprises one Hydrogen atom and one Bromine atom. If you wonder if this molecule is polar or nonpolar, well, HBr is a polar molecule. Wondering how and why? This detailed blogpost on Hydrogen Bromide Polarity will help you determine if the molecule is polar or nonpolar.
HBr Polarity
One can determine the polarity of any given molecule by considering various factors that contribute to the molecule’s chemical properties. These factors are:
- Lewis structure of the molecule
- The shape of the molecule
- The difference in electronegativities of the atoms in the molecule
- Net dipole moment in the molecule
The HBr molecule has a simple Lewis structure, as there is one Hydrogen atom forming a covalent bond with a Bromine atom. As the Hydrogen atom is a group 1 element, it needs one more valence electron to attain a stable structure. In contrast, the Bromine atom has seven valence electrons in its outer shell and requires one more valence electron to complete its octet.

Here in HBr, both these atoms share two valence electrons by forming a single bond. Hence the octets of both these atoms are complete, and it has a stable structure.
When we look at the shape of the molecule, it is linear as it is a diatomic molecule. The molecules that have two atoms generally have a linear geometry.
Now, here is the crucial part which helps us know if the molecule is polar or nonpolar. The difference in electronegativities of the atoms helps us find out if the bond is polar or not. When the difference of electronegativities for the atoms in the molecule is higher than 0.4, the bond is considered a polar bond.
And in a polar bond, the atom with the most electronegativity values has a pull on shared electrons, leading to uneven distribution of charges. However, if the difference is lower than 0.4, the valence electrons in the bond are shared equally, and there is no uneven distribution of charges.
For the Hydrogen Bromide molecule, we have to consider the electronegativities of both Hydrogen and Bromine atoms.
Why is HBr a polar molecule?
The hydrogen atom has an electronegativity value of 2.2, while the electronegativity value for Bromine is 2.96. If you calculate the differences of electronegativities for these atoms, it is higher than 0.4.
As the Bromine atom in HBr is more electronegative than the Hydrogen atom, it will pull the shared electrons towards itself.
Due to the bond’s polarity, there will be a net dipole moment in the molecule. The vector of the dipole moment will be from the Hydrogen atom to the Bromine atom. As a result of the uneven distribution of charges, the regions around the Bromine atom have partial negative charge and regions around the Hydrogen atom have partial positive charges.
Due to the formation of poles, the negatively charged pole and positively charged pole are formed in the molecule, and as a result, HBr or Hydrogen Bromide is a polar molecule.
Concluding Remarks: Is HBr polar or nonpolar
To summarize this blog post, in a nutshell, we can say that Hydrogen Bromide or HBr is a polar molecule because of the high electronegativity differences of Hydrogen and Bromine atoms. As Bromine is more electronegative, it tries and pulls the shared valence electron towards itself, which leads to the net dipole moment in the molecule. This nonzero dipole moment in HBr gives a partial positive charge to Hydrogen and a partial negative charge to Bromine.
And hence, due to the uneven distribution of charges, HBr is a polar molecule. Other molecules such as HCl, NH3, OF2, etc are also polar molecules due to uneven distribution of charges.