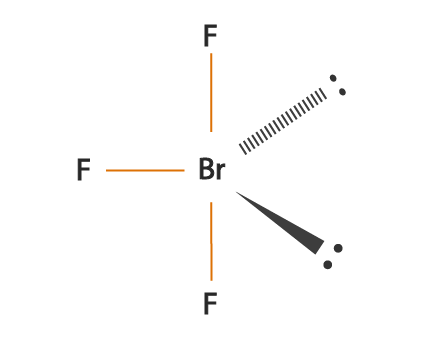
Bromine Trifluoride is commonly used as a strong fluorinating agent as it is a strong interhalogen compound. Both Bromine and Fluorine are halogens. This compound usually exists in a liquid form and has quite a pungent odor. The chemical formula for this compound is BrF3. The compound was first discovered in 1906 by Paul Lebeau by carrying out Bromine and fluorine’s reaction at 20 degrees celsius.
It forms a T-shaped molecular structure and has Bromine element as the central atom. To know more about its physical properties, chemical properties, and uses, it’s vital first to understand the geometry of the molecule and its hybridization, polarity, etc.
| Name of molecule | Bromine Trifluoride |
| No of Valence Electrons in the molecule | 28 |
| Hybridization of BrF3 | sp3d hybridization |
| Bond Angles | 86.2 degrees |
| Molecular Geometry of BrF3 | Trigonal Bipyramidal |
Contents
BrF3 electron geometry
BrF3 is a perfect example of an AX5 molecule with two lone pairs of electrons and three bonded pairs of electrons. Each fluorine atoms has nine electrons, and there are seven valence electrons in the outer shell of the Bromine molecule, out of which three electrons form bonds with three fluorine atoms. This results in three bonded pairs of electrons and two lone pairs.
According to the VSEPR theory, the molecular shape of the molecule should be trigonal pyramidal. Still, to minimize the repulsion between the lone pairs, there is a bent in its shape, which makes this molecule T-shaped.

BrF3 Hybridization
To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. It is represented as 1s2 2s22p6 3s23p63d104s24p5.
However, to form bonds with the fluorine atoms, some electrons in Bromine are shifted to 4d-orbitals. This is possible because fluorine has a higher oxidative capacity, and hence it forces Bromine to promote electrons to the said level. Now, Bromine can use the d-orbitals for hybridization.
BrF3 consists of seven electrons in its outermost shell. After the bond formation, it will further have two lone pairs and 3 Br—F covalent bonds (bonding pairs). As the hybridization value or the electron pair is equal to 5, it gives rise to sp3d hybrid orbitals. Hence its hybridization is sp3d.
So the hybridization of the BrF3 molecule is sp3d.
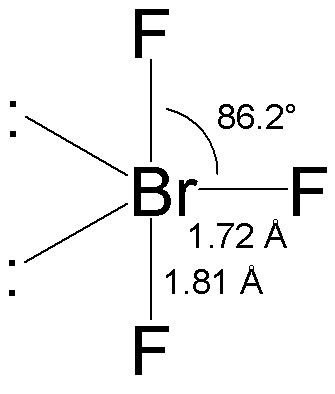
BrF3 Bond angle
BrF3 molecular geometry is said to be T-shaped or trigonal bipyramidal (as discussed) with a bond angle of 86.2°, which is slightly smaller than the usual 90°. The angle is formed due to the electron pairs’ repulsion, which is greater than that of the Br-F bonds. [The compressed bond angles with respect to a perfect trigonal bipyramid are due to lone pairs spreading out more in space than bonded pairs.]
Is BrF3 polar?
The bonds of Br-F are considered polar because of a relatively high difference in electronegativity values of fluorine and bromine atoms in the compound. The unshared pairs or the lone pairs are located in the plane of the triangle, causing an uneven distribution of negative charge around the central bromine atom and, in turn, makes the compound highly polar. Thus one can say that Bromine trifluoride is polar.

Concluding Remarks
Bromine Trifluoride or BrF3 is a strong fluorinating agent, and its central atom has sp3d hybridization. It is a T-shaped molecule with a bond angle of 86.2°. The molecular is highly polar and is majorly used for the production of uranium hexafluoride. I hope this article helps you understand the molecular geometry of BrF3 along with its other properties.