The chemical formula BeH2 represents Beryllium Hydride. The compound exists in both a gaseous (dihydridoberyllium) and solid state. It cools down to polymerize into a colourless amorphous solid represented by the chemical formula (BeH2)n.
Unlike other Group 2 metals that form ionic bonds, Beryllium forms covalent bonds with the Hydrogen atoms present. Beryllium (Be2+) forces the incoming Hydrogen (H-) anions to form covalent bonds, which means that the two elements are not interacting electrostatically.
Synthesis of the compound isn’t straightforward as, unlike other group 2 metals; Beryllium does not react with Hydrogen. It can be obtained by the reaction of dimethylberyllium [Be(CH3)2] with lithium aluminium hydride, LiAlH4.
Purer samples can be obtained through pyrolysis of di-tert-butylberyllium, Be(C(CH3)3)2 at 210 °C, and the reaction of triphenylphosphine, PPh3, with beryllium borohydride, Be(BH2)2.
Beryllium Hydride decomposes slowly with water. However, the reaction of BeH2 with Hydrogen Chloride rapidly produces Beryllium Chloride.
BeH2 + 2 H2O → Be(OH)2 + 2 H2
BeH2 + 2 HCl → BeCl2 + 2 H2
Beryllium hydride reacts with Lewis bases such as trimethylamine, N (CH3)3, to form dimeric adducts containing bridging hydrides.
Research suggests that BeH2 could represent a solution to molecular Hydrogen storage. Decomposition of the polymer releases Hydrogen gas that could potentially be used to fuel rockets.
Some of the properties of BeH2 are given below:
| Name of the molecule | BeH2 |
| No. of valence electrons | (2 x 1) + (1 x 2) = 4 valence electrons |
| Hybridization of the central atom | Sp |
| Bond Angles | 180° |
| Molecular Geometry of BeH2 | Linear |
Contents
BeH2 Valence Electrons
As mentioned in previous articles, the Lewis structure of a compound necessitates the calculation of Valence electrons available.
Valence electrons are used to form chemical bonds and take part in exchanges. They play a crucial role in determining various other properties, forming the basis of the VSEPR theory.
In this case, BeH2 comprises an alkaline metal, i.e., Beryllium and Hydrogen.
Beryllium is in group 2 of the periodic table alongside other alkali metals. Its electronic configuration is given by [He] 2s². Beryllium has two electrons in its outermost shell. Therefore, by definition, the lone Beryllium atom in BeH2 contributes 2 x 1 = 2 valence electrons.
Hydrogen, as we know, has an electronic configuration of 1s1. Therefore, the two Hydrogen atoms contribute 1 x 2 = 2 valence electrons.
Therefore, the total number of valence electrons available to use from Beryllium Hydride:
2[Be] + 2[H] = 4 Valence Electrons
BeH2 Lewis Structure
The Lewis structure is the schematic arrangement of the constituent atoms, chemical bonds, and valence electrons of a particular compound.
In this case, the compound in focus is Beryllium Hydride. The compound comprises two Hydrogen atoms and a Beryllium atom.
The Lewis structure necessitates that we calculate the number of valence electrons available. This has been addressed in the previous section. The number of valence electrons available is proven to be 4. We can now form covalent bonds and fulfil octet requirements using these four valence electrons.
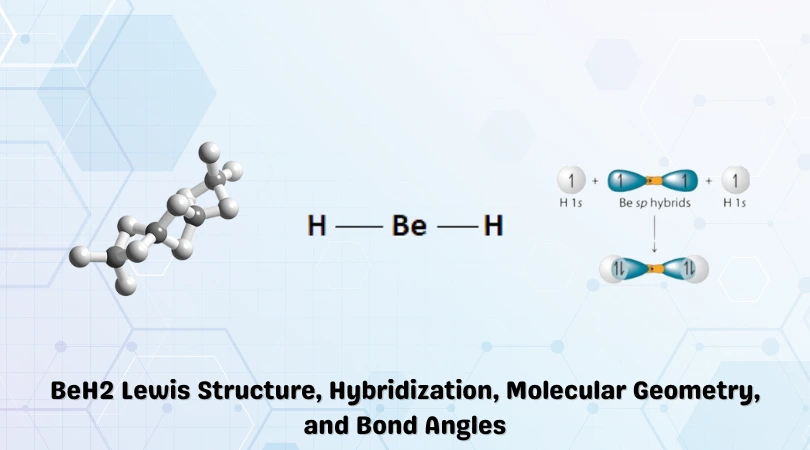
Beryllium is the central atom with the two Hydrogen atoms placed on either flank. This is shown in the figure below:
Next, we place two valence electrons between atoms to form covalent bonds. As mentioned in the introductory section, Beryllium does not form ionic hydrides, unlike other groups 2 elements (MgH2, CaH2, BaH2). The chemical bond between Hydrogen and Beryllium is covalent. This is shown below.
Hydrogen’s outer shell requirements have been filled, considering that it only requires a duplet. Beryllium, on the other hand, is a curious case. The electronic configuration suggests that the octet requirements aren’t fulfilled. After all, only four valence electrons are available to us. Like Hydrogen and its duplet, Beryllium is also an exception to the octet rule.
Therefore, the Lewis structure for Beryllium Hydride shown below is stable.
BeH2 Hybridization
Hybridization of the BeH2 molecule can be explained using the steric number/electron domain concept as well as the valence bond theory.
There are two covalent bonds present in the BeH2 molecule, and this results in a steric number of 2. As such, the hybridization of Beryllium Hydride will be sp.
Using the Valence Bond Theory, we can establish a more detailed look at how this sp hybridized state comes to be. In its excited state, one of the electrons in the 2s2 orbital jumps to a 2p orbital.
Hybridization results in two sp orbitals with equal energies resulting from the fusion of the 2s and 2p orbitals. These sp orbitals in Beryllium overlap with 1s orbitals of the adjacent hydrogen atoms.
This verifies the sp nature of hybridization in BeH2.
BeH2 Bond Angles
According to the VSEPR theory, the Hydrogen atoms repel each other. As such, the bond angle of BeH2 is 180° resulting in a linear shape.
BeH2 Molecular Geometry
To determine the molecular geometry of BeH2, we must observe the Lewis structure determined earlier. In this structure, two Hydrogen atoms are separated by a Beryllium atom in the middle.
According to the VSEPR theory, these two Hydrogen atoms want to get as far away from each other as possible. They repel each other, and we can visualize a linear shape forming.
We can also determine this using a steric number or A-X-N method chart. A steric number of 2 corresponds to a linear shape. The same is true for an AX2 notation with zero lone pairs.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
However, this only remains true for the gaseous state. As there are empty 2p orbitals present, the gas cools down to form an amorphous solid that turns crystalline in extreme conditions—the polymerization results in a tetrahedral shape being formed.
A complex 3D web is constructed, which can be better explained in detail by the Molecular Orbital theory.
For this article, however, we shall consider the molecular geometry of BeH2 to be Linear.
Concluding Remarks
Let’s quickly summarize the features and properties of Beryllium Hydride
- Beryllium Hydride is a unique Hydrogen Storage solution.
- Beryllium acts as the central atom. Unlike other Group 2 elements, Beryllium forms covalent bonds with Hydrogen.
- The hybridization of BeH2 is given by sp because there are only two covalent bonds.
- BeH2 in its gaseous form has a linear molecular structure with bond angles of around 180°. It cools down to polymerize and forms complex 3D structures.
BeH2 is an electron-deficient molecule and thus acts as a Lewis acid.