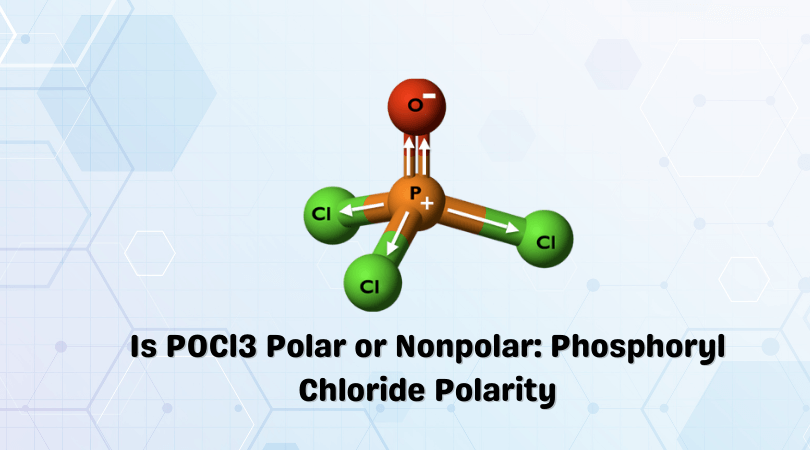
Phosphoryl chloride or Phosphorus Oxychloride is an inorganic compound that exists in colorless liquid. The compound hydrolyses in the presence of moist air and forms phosphoric acid. The molecule has a tetrahedral shape and is composed of three atoms: Phosphorus, Chlorine, and Oxygen.
Phosphorus forms a double bond with Oxygen and a single bond with all the Chlorine atoms. You can check in lewis structure. Many readers get confused when it comes to the polarity of POCl3 due to the arrangement of atoms and the shape of the molecule.
The polarity of any molecule depends on various factors such as the shape of the molecule, distribution of valence electrons, and difference in electronegativities of the atoms in the molecule.
There is a difference in the electronegativities of all three atoms. Phosphorus is the least electronegative atom, whereas Oxygen is the most electronegative atom. The difference in electronegativities for Phosphorus and Oxygen as well as Phosphorus and Chlorine is higher than 0.4. As a result, there is a dipole moment in the molecule.
When you look at the shape of the molecule, it might seem symmetric as the central atom forms bonds with four other atoms. Here, the central atom forms a double bond with Oxygen and single bonds with other atoms that make the arrangement of the molecule asymmetrical. And as the shape of the molecule is not symmetric, the dipole moments will not cancel each other. Hence, there is a net dipole moment in the molecule.
Is POCl3 polar or nonpolar?
Phosphoryl Chloride or Phosphorus Oxychloride is a polar molecule due to the uneven distribution of valence electrons in the molecule and a net dipole moment in it. Due to the asymmetry seen in the molecule’s shape, the dipole moments are not nullified, making this molecule a polar molecule. The bonds between Phosphorus and Oxygen are moderately covalent.
Uses of POCl3
- This compound is used in laboratories for a range of dehydration reactions.
- POCl3 is also used as a source of liquid phosphorus in diffusion processes for semiconductor industries.
- It also plays an imperial role in the Vilsmeier-Haack reaction as it is used to prepare Vilsmeier’s reagent.
- Phosphoryl Chloride is used in industries for manufacturing phosphate esters, e.g., it is used in the synthesis of Triphenyl phosphate.