A lot of chemistry students tend to get confused between exothermic and exergonic reactions. This happens because both of these reactions do not need energy or heat to carry out their reactions. However, both these reactions differ from each other. In this blog, we will look at the differences between these reactions, but first, let us go through the definitions of these reactions.
Exothermic Reactions: “Exo” means exit and thermic means heat, which means the heat energy is released in this reaction, hence the name exothermic. The chemical reaction in which there is a standard change in the enthalpy is termed as exothermic reactions. These reactions release energy in its surroundings, and there is a change in the temperature of the surroundings.
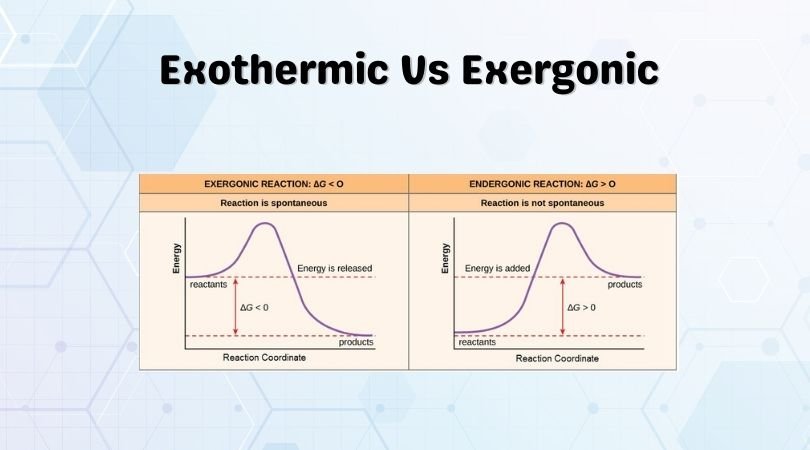
Exergonic Reactions: The chemical reactions that occur in favourable conditions spontaneously without needing any energy or heat are known as exergonic reactions. The change of free energy in this reaction is negative, and there is no change in the temperature of the system after the reaction. The initial and final temperatures of the system are the same in exergonic reactions.
Exothermic vs Exergonic
Exothermic Reactions |
Exergonic Reactions |
| Exothermic reactions are the ones that release heat or energy in one or another form. | These types of reactions occur in favorable conditions spontaneously. |
| Although these reactions are similar to exergonic reactions, there is a change in the enthalpy of the reaction and not in the Gibbs free energy of the reaction. The overall change in the enthalpy is negative for such reactions. | There is a decrease in free energy during this reaction, leading to a decreased Gibbs free energy, and it is less than zero. |
| The entropy (S) of the systems increases in exothermic reactions, which means the randomness increases in the reaction. | The entropy (S) of the system increases during such reactions. |
| The energy released in these reactions is in the form of heat or light such as sparks, explosions etc. | There is a flow of free energy from the system to its surroundings. |
| An exothermic reaction is an exergonic reaction because the change in enthalpy will also lead to the difference in the Gibbs free energy of the system. | Although there is a release of heat in this reaction, the temperature of the surroundings does not increase. If there is an increase in the temperature of the surroundings, then it becomes an exothermic reaction. |
| Combustion of wood or coal is an apt example of an exothermic reaction. | Breakdowns of sugar is an example of an exergonic reaction. |
| Exothermic reactions also increase the temperature of the surroundings. | These types of reactions do not need energy; instead, it releases energy in the surroundings. |
Concluding Remarks
To conclude, we can say that there is a change in standard enthalpy in exothermic reactions and a change in the Gibbs free energy in the exergonic reactions. The reactions that increase the heat of the surroundings are classified as exothermic reactions, whereas the ones that do not change the temperature of the surroundings are exergonic. Both these reactions do not require additional energy or heat to complete their reactions.