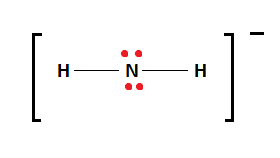
The chemical formula NH2– represents the Azanide ion. Azanide is the IUPAC name of the ion, however this nomenclature is obscure. The derivatives of the NH2– ion are referred to as amides. The term ‘amide’ also refers to the –C(O)NR2, which is an organic functional group.
Unlike its organic counterpart, the Azanide ion is a conjugate base of Ammonia. Deprotonation of Ammonia occurs when it reacts with strong bases or alkali metals. This leads to the formation of the Azanide anion.
Alkali metal derivatives (also known as alkali metal amides) such as Sodium Amide, Lithium Amide and Potassium Amide, are produced when liquid Ammonia is treated with strong bases. A schematic equation of this reaction is shown below:
2 M + 2 NH3 → 2 MNH2 + H2 (M = Li, Na, K)
We say that the Azanide ion is polar. Let’s see why that is the case.
To determine the polarity of the NH2– ion, we must first account for its properties. These include its electronegativity, its molecular geometry and its resulting dipole moment, if any.
NH2- Electronegativity and Bond Nature
The difference in electronegativity generates regions of electropositive and electronegative in the ion or molecule contributing to its polar nature.
We look to the periodic table to determine the difference in charges between the Nitrogen and Hydrogen atoms. In this case the difference is 3.04 [N] – 2.20 [H] = 0.84.
Now, we look to the Pauling scale to help determine bond nature and polarity. According to the Pauling scale, if the difference electronegativity lies between 0.5 and 2.0, the bonds are said to be polar in nature.
Since that is the case in Azanide ion, the N-H bonds are said to be polar. This indicates polar nature. Let’s examine further.
NH2- Molecular Shape and Symmetry
The NH2– ion has two Hydrogen atoms bonded to the central Nitrogen atom as shown in the figure. The central Nitrogen atom also possesses two lone pairs.
According to the VSEPR theory the Hydrogen atoms repel each other and spread far away in an even manner. The two lone pairs make the structure unsymmetrical.
The ion has bond angles of 104.5°.
NH2- Net Dipole Moment and 1- Charge
The 1- charge over the entire molecule is distributed evenly. It interacts with polar solvents such as water due to this charge.
Due to the presence of lone pairs on the central Nitrogen atom, there is a net dipole moment. This is also due to its bent molecular structure. The dipole vectors converge and sum up.
This in turn generates region of electropositivity on the outer Hydrogen atoms. The central Nitrogen atom gains a partial negative charge.
Conclusion
As discussed above, the Azanide ion is unsymmetrical. The it also possesses N-H bonds which are polar in nature due the difference in electronegativity between Hydrogen and Nitrogen.
This combined with a net dipole moment squarely indicates that NH2– is polar in nature.