Polarity is a property that is observed in compounds having the separation of positive and negative charges in any given compound. This happens when there is quite a considerable difference between the electronegativities of the atoms in the molecule. As polarity helps determine other physical properties of the molecule, it is essential to determine if the molecule is polar. In this blog, we will look at the polarity of CH3Cl and find out if the molecule is polar or not.
Polarity of CH3Cl
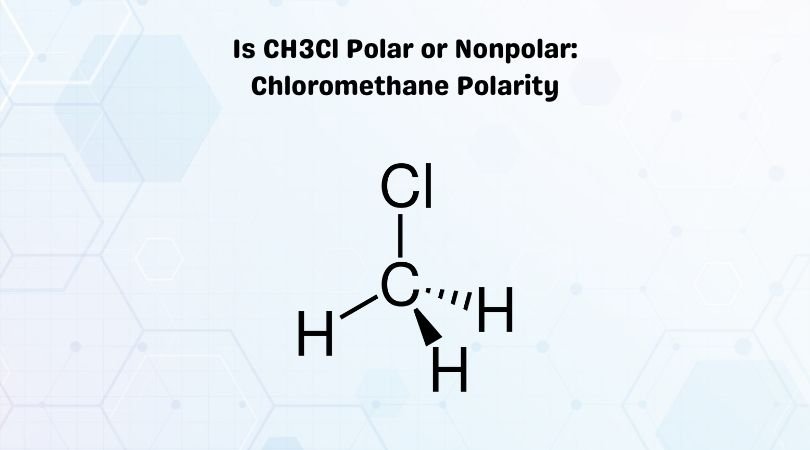
Chloromethane, having a chemical formula CH3Cl, has one atom of Carbon in its central position, three hydrogen atoms, and one atom of Chlorine. As Carbon is less electronegative than Chlorine, it takes a central position, and rest all atoms are arranged around it.
When you look at the Lewis dot structure of this molecule, there are no lone pairs of electrons in its structure. Carbon shares its four electrons, which completes the octet of all the atoms in the molecule. In total, there are 14 valence electrons of all the three atoms that participate in forming bonds giving a tetrahedral shape to the molecule.
Suppose you compare the electronegativities of both the atoms carbon and Chlorine. In that case, you will find out that the chlorine atom is more electronegative than the carbon atom as it is closer to Flouirne on the periodic table. So there will be a dipole moment between Chlorine and Carbon atom. As Chlorine has more electronegativity, it tries to pull the electrons on its side. This dipole moment results in the unequal distribution of charges on Carbon and Chlorine. Thus C-Cl bond is considered to be polar.
When it comes to the bond between Carbon and Hydrogen atoms, the difference in the electronegativities of both these atoms is relatively small. Due to which the C-H bond is considered nonpolar.
However, as there are partial negative charges on the Chlorine atom and have a net dipole moment, CH3Cl is a polar molecule.
Physical properties of Chloromethane
Methyl chloride or Chloromethane is an odorless and flammable gas. It is an organic compound. It is a colorless gas, and it also has a faintly sweet odor, which is observed only at a certain level where it is quite toxic.
| Name of the molecule | Chloromethane |
| Molecular Formula | CH3Cl |
| Molecular weight | 50.49 g/mol |
| Boiling point | -23.8℃ |
| Melting point | -97.4 ℃ |
Uses of Chloromethane
- Chloromethane was used as a refrigerant but was discontinued later when it found that the gas has harmful effects.
- Methyl chloride is also used as a methylating and chlorinating agent in chemical industries.
- This compound is also used as a herbicide and as a local anesthetic as well.
- Many industries also use this gas as a catalyst carrier of the polymerization process at low temperatures.
- It is also used as an extractant for oils, greases, and resins.
Concluding Remarks
Electronegativity plays a vital role in deciding whether the given molecule is polar or nonpolar. Here is CH3Cl, as Chlorine has more electronegativity than all the other atoms, it has a partial negative charge, and the hydrogen atoms have partial positive charges. This net dipole moment in the molecule makes Chloromethane a polar molecule.