One of the most frequently asked questions among the students studying chemistry is regarding the polarity of Carbon Monoxide. Although its structure is easy to understand, people still get confused when asked if CO is polar or nonpolar. So here is the answer you have been looking for: Carbon Monoxide is a polar molecule. Wondering how? Well, I have listed all the reasons in this blog post, so read it and find out why CO is a polar molecule.
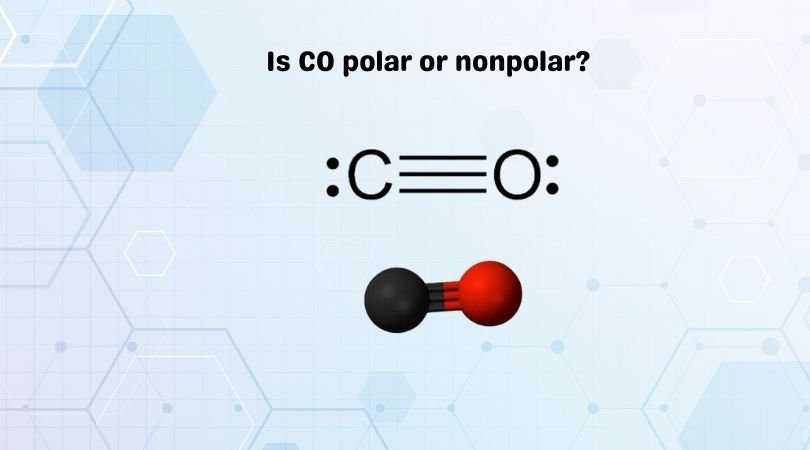
Carbon Monoxide or CO is a diatomic molecule. Here is the Lewis structure for the molecule.
Carbon and Oxygen atoms share a triple bond to fill their octets. Both the atoms have lone pair of electrons and are sharing other electrons to complete their octets. It has a linear molecular geometry, given that there are only two atoms in this molecule. The arrangement of these lone pairs is symmetric, and that’s the reason people get confused.
But apart from the electrons’ arrangement, it is vital to check the dipole moment between the atoms. And for knowing if there is a dipole moment in the molecule, we need first to see the electronegativities of both the atoms and check if there is a difference between them.
Carbon is a group 14 element on the periodic table having an electronegativity of 2.55, whereas Oxygen is a group 16 element having an electronegativity of 3.44. If you notice, the difference between both Carbon and Oxygen atoms is higher than 0.5, which makes the bond between Carbon and Oxygen polar.
The oxygen atom is slightly higher in electronegativity, and hence it will try to pull the shared electrons to its side. This dipole moment in the molecule makes CO a polar molecule. The higher electronegativity if Oxygen makes it partially negatively charged and Carbon partially positively charged. And such formation of partial charges leads to the polarity in the molecule.
However, some people still believe that the molecule is nonpolar practically. But looking at the difference in the electronegativities and polarisation in this molecule, we can say that Carbon Monoxide is a polar molecule.