The XeF4 or Xenon Tetrafluoride is a chemical compound made of Xenon and Fluoride atoms. It is the world’s first binary compound discovered. It is a type of noble gas having the chemical equation of
Xe +2 F2 -> XeF4
The XeF4 has a solid white appearance and has a density of 4.040 g cm−3 in a solid form. Under ordinary conditions, it appears like a colorless crystalline. It has a sublime temperature of 115.7-degree Celsius or 240.26-degree Fahrenheit. Same as the other Xenon Fluorides, the Xenon Tetrafluoride has an exergonic formation. At normal temperature and pressure, it stays in stable condition. It reacts with water instantly and releases molecular oxygen, hydrogen fluoride, and pure xenon gas.
| Name of molecule | Xenon Tetrafluoride (XeF4) |
| No of Valence Electrons in the molecule | 36 |
| Hybridization of XeF4 | sp3d2 hybridization |
| Bond Angles | 90 degrees and 180 degrees |
| Molecular Geometry of XeF4 | Square Planar |
To know more about its physical properties and chemical properties, one needs to know its Lewis structure and molecular geometry. Let us find out the Lewis structure of Xenon tetrafluoride.
For making the Lewis structure, we need to know the valence electrons of XeF4 to make its structure and know the placement of atoms in the molecule.
Contents
XeF4 Valence electrons
In this molecule, we have one atom of Xenon and four atoms of Fluorine. We will calculate the valence electrons of both these atoms to determine the total number of valence electrons of XeF4.
Valence electrons of Xenon = 8
Valence electrons of Fluorine = 7*4 ( as there are four Fluorine atoms, we will multiply it by 4)
Total number of valence electrons of Xef4: 8 + 7*4
: 8 + 28
: 36
Hence there are a total of 36 valence electrons in XeF4.
XeF4 Lewis Structure
Now that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to draw its Lewis structure. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms.
The bonds in the structure are shown using lines, whereas the electrons not participating in the bond formation are shown as dots. Electrons that do not form any bond are called nonbonding electrons or lone pairs of electrons.
Here as Xenon is the least electronegative atom, we will place it in the center and all the other fluorine atoms around it like this:
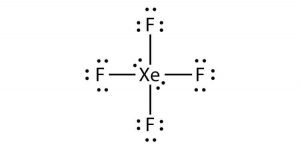
Now that we have placed all the atoms let’s show bonds between each Fluorine and Xenon atom. Each bond in the molecule takes up two electrons, and as there are four single bonds in this molecule, 8 electrons out of 36 are used up.
Start placing the rest of the valence electrons around the atoms. Each fluorine atom will have six valence electrons around it, as one electron was used to make the bond.
You might notice that we have already placed 24 electrons out of 28 around the fluorine atoms. The remaining nonbonding electrons or lone pairs of electrons will be placed on Xenon as it is an exception to the octet rule.
Place these two pairs of nonbonding electrons on Xenon, and now you have a Lewis structure where there are two lone pairs of electrons on Xenon and six nonbonding electrons on each Fluorine atom.
XeF4 Hybridization
The central Xenon atom’s orbitals are hybridized, which results in the formation of new hybridized orbitals. Xenon has six electrons in its 5p orbitals and two electrons in 5s orbitals. There are no electrons in d-orbitals and f-orbitals in the ground state of Xenon. But when this atom is in an excited state, two electrons in the p-orbitals move to d-orbitals; as a result, there are four unpaired electrons in total. Out of which, two are in p-orbitals, and the other two unpaired electrons are in d-orbitals. These hybridized orbitals lead to sp3d2 hybridization in XeF4.
XeF4 Molecular Geometry
It is easier to understand the molecular geometry of a given molecule once we know its Lewis structure. As Xenon has two lone pairs of electrons, it will take up a structure that helps these lone pairs avoid the repulsion forces. To keep these repulsions at a minimum, the lone pairs will be in a perpendicular plane. And as there are four fluorine atoms, the molecule will have an arrangement such that its molecular geometry is square planar. XeF4 has an electronic geometry of octahedral, making the molecular geometry of Xenon Tetrafluoride square planar.
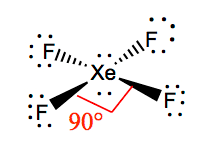
XeF4 Bond angles
The bond angles of F-Xe-F are 90 degrees, and lone pairs have angles of 180 degrees. The Fluorine atoms are located at 90 degrees to each other, resulting in the symmetric distribution of the electrons in the molecule’s plane. These bond angles contribute to the formation of square planar molecular geometry.
XeF4 Polarity – Is XeF4 Polar or Nonpolar?
Although the bonds between Xenon and Fluorine atoms are polar, XeF4 is a nonpolar molecule. Wondering how? All the Xe-F bonds are in opposition with each other mutually, making the sum of dipole moment zero. As there are four electrons on the Xenon atom, which are localized as nonbonding pairs of electrons. As the overall arrangement of the atoms and electrons in the molecule is such that the vector sum of the dipoles is zero, XeF4 is a nonpolar molecule.
Concluding Remarks
Xenon Tetrafluoride is one of those molecules that is relatively easy to understand. Its Lewis structure is one of the least complicated structures, as all the Fluorine atoms are arranged in the symmetric pattern. The lone pairs in the molecule are located in a perpendicular plane in an octahedral shape to keep their repulsive forces at a minimum.
To summarize this blog post, we can say that XeF4 has 36 valence electrons. It has two lone pairs of nonbonding electrons on the central atom of Xenon. The molecule has octahedral electron geometry and square planar molecular geometry. XeF4 is a nonpolar molecule and has sp3d2 hybridization.
At the Geometry of Molecules, we like knowing what you think. So let us know your thoughts on this molecule in the comments below.

HI JANICE,
Nice explanation. I have found many posts on XeF4, but this post contains all what I want. I want to know that how can we calculate the formal charge of an atom. Also, how to decide the structure of the molecule i.e. symmetric or asymmetric.
Thanks
Good explanation about XeF₄
Thank You. Keep Reading!