Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton. In this blog post, we will learn about the Lewis dot structure, electron geometry, and molecular geometry of this molecule.
| Name of molecule | Ammonia / Nitrogen Trihydride ( NH3) |
| No of Valence Electrons in the molecule | 8 |
| Hybridization of NH3 | sp3 hybridization |
| Bond Angles | 107 degrees |
| Molecular Geometry of NH3 | Trigonal Pyramidal |
And to understand the Lewis structure, we first need to find out the valence electrons in this molecule. Electrons in the atom’s outermost shell are termed as valence electrons and are vital for as they are responsible for forming bonds as well as the structure of the molecule.
Contents
Valence electrons of NH3 ( Ammonia )
Nitrogen is a group 15 element and has five electrons in its outer shell. In contrast, Hydrogen is a group 1 element and only has 1 valence electron in its outer shell. To get the total number of valence electrons, we will add up the valence electrons for both these atoms.
Nitrogen – 5 valence electrons
Hydrogen – 1 electron, but as there are 3 Hydrogen atoms we will multiply it by 3, there are three valence electrons of all Hydrogen atoms.
Total number of valence electrons – 5+3
= 8 valence
Ammonia or NH3 has a total of 8 valence electrons.
NH3 Lewis Structure
The Lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity, and other such properties with ease. It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. The electrons that form bonds are called bonding pair of electrons, whereas the ones that do not form any bonds are called nonbonding pairs of electrons or lone pairs of electrons.
Dots are used to show the valence electrons, whereas the lines represent bonds in the structure. Here is the step-by-step procedure to understand the Lewis structure of NH3.
Now that we know the valence electrons for the molecule, we can predict its Lewis structure. Hydrogen atoms never take the central position, so we will place the Nitrogen atom in the center.
Place all the Hydrogen atoms around the Nitrogen atom and the valence electrons of both the atoms like this.
Each Hydrogen atom only needs one electron to become stable, as it is an exception to the octet rule. Nitrogen will share three of its valence electrons for forming a stable structure.
Thus there are three single bonds formed between Nitrogen and Hydrogen atoms, and there is one pair of nonbonding electrons on the nitrogen atom.
NH3 Molecular Geometry
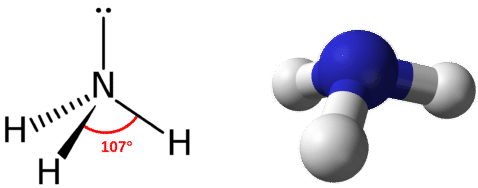
Ammonia has a tetrahedral molecular geometry. All the Hydrogen atoms are arranged symmetrically around the Nitrogen atom which forms the base, and the two nonbonding electrons form the tip which makes the molecular geometry of NH3 trigonal pyramidal.
NH3 Hybridization
The Nitrogen atom has the electronic configuration of 1s2 2s2 2px1 2py1 2pz1. When it shares the electrons with Hydrogen atoms, one s-orbital and three p-orbitals hybridize and overlap with s orbitals of a Hydrogen atom to form sp3 hybridization.
Thus, Ammonia or NH3 has sp3 hybridization.
NH3 Bond angles
There are three single bonds and one lone pair of electrons in the NH3 molecule. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. The shape is distorted because of the lone pairs of electrons. This pair exerts repulsive forces on the bonding pairs of electrons. Although the bond angle should be 109.5 degrees for trigonal pyramidal molecular geometry, it decreases to 107 degrees due to the lone pair on the nitrogen atom.
Concluding Remarks
Ammonia is a stable binary hydride having a Trigonal Pyramidal molecular geometry and sp3 hybridization. It has bond angles of 107 degrees and an electron geometry of tetrahedral. To know more about its polarity, read our blog on polarity.

Good
Thank you very much mam
It was really very much helpful
Thank you so much!
Your Welcome