Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds.
And to further understand Hydrogen Cyanide’s physical properties, it is vital to know its Lewis structure and molecular geometry. Keep reading this post to find out its shape, polarity, and more. First, let us look at its Lewis dot structure and the valence electrons that participate in forming bonds.
Contents
HCN valence electrons
To draw the Lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. To know the valence electrons of HCN, let us go through the valence electrons of individual atoms in Hydrogen Cyanide.
This molecule is made up of three different atoms: Hydrogen, Carbon, and Nitrogen.
Hydrogen has one valence electron, and it only needs one more electron to complete its valence shell as it is an exception to the octet rule.
So Hydrogen has one valence electron.
Whereas Carbon has four valence electrons and Nitrogen has five valence electrons.
Total number of valence electrons in HCN= No. of valence electrons in Hydrogen + No. of valence electrons in Carbob+ No.of valence electrons in Nitrogen
= 1+4+5
= 10 valence electrons
Hence, Hydrogen Cyanide, HCN, has ten valence electrons.
HCN Lewis structure
Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule. It also aids with understanding the bonds formed in the molecule and the electrons not participating in any bond formation.
To start with making the Lewis Structure of HCN, we will first determine the central atom. And then place the remaining atoms in the structure.
As Carbon is the least electronegative atom in this molecule, it will take the central position. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this:
Once you have arranged the atoms, start placing the valence electrons around individual atoms. Like Hydrogen will have one electron, Carbon will have four electrons, and Nitrogen will have five electrons around its atom like this:
If you look at the structure closely, you will realize that Hydrogen can share one electron with the Carbon atom and become stable. So both Carbon and Hydrogen will share two electrons and form a single bond.
H-C N
Now that we have completed the valence shell for Hydrogen let us do the same for the Carbon atom. The atom is left with only three valence electrons as it has shared one electron with Hydrogen. And so Carbon will share its remaining three electrons with Nitrogen to complete its octet, resulting in the formation of a triple bond between Carbon and Nitrogen.
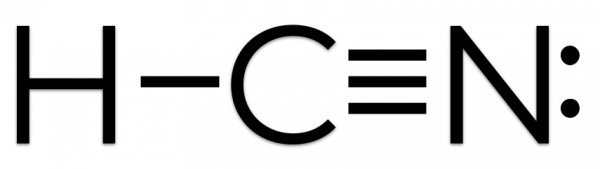
Carbon has a complete octet by forming a single bond with Hydrogen and a triple bond with the Nitrogen atom. Similarly, Nitrogen has a complete octet as it only needed three electrons for completing the octet that it got by sharing the electrons with Carbon. Hydrogen has two electrons in its outer valence shell. The rest two electrons are nonbonding electrons.
HCN Molecular Geometry
The molecular Geometry of any given molecule helps understand its three-dimensional structure and the arrangement of atoms in a molecule, and its shape. Hydrogen Cyanide has geometry like AX2 molecule, where A is the central atom and X is the number of atoms bonded with the central atom.
As Carbon is bonded to two atoms, it follows the molecular geometry of AX2. And as per VSEPR theory, molecules covered under AX2 have a linear molecular geometry.
Hence Hydrogen Cyanide has linear molecular geometry.
HCN Bond Angles
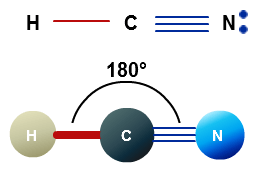
Once we know the Lewis structure and Molecular Geometry of any molecule, it is easy to determine its bond angles and polarity. As this molecule has a linear molecular geometry, HCN has bond angles of 180 degrees.
HCN Shape
As both Hydrogen and Nitrogen are placed far from each other at bond angles of 180 degrees, it forms a linear shape.
HCN Polarity
HCN in a polar molecule, unlike the linear CO2. And here is why:
Carbon has an electronegativity of 2.5, Hydrogen’s electronegativity is 2.1, and Nitrogen has an electronegativity of 3.
Although Hydrogen is the least electronegative, it can never take a central position. And due to the difference in electronegativities between Carbon and Hydrogen, the vector represents charge will be drawn from Hydrogen to Carbon. Similarly, as Nitrogen is more electronegative than Carbon, the vector will be towards Nitrogen from Carbon.
Despite quite a small difference in Carbon and Nitrogen’s electronegativities, it is considered a slightly polar bond as Nitrogen will try to pull the electrons to itself. Due to such differences, Hydrogen will have slightly positive charges, and Nitrogen will have slightly negative charges as the vector goes from Hydrogen to Nitrogen.
Thus Nitrogen becomes a negative pole, and the Hydrogen atom becomes a positive pole, making the molecular polar. Any molecule that has a difference of electronegativities of any dipole moment is considered as polar.
Hence, Hydrogen Cyanide is a polar molecule.
Concluding Remarks
To summarize everything in this article, we can say that:
- Carbon forms one single bond with the Hydrogen atom and forms a triple bond with the Nitrogen atom.
- HCN has a total of 10 valence electrons.
- It is covered under AX2 molecular geometry and has a linear shape.
- The bond angles of HCN is 180 degrees.
- Hydrogen Cyanide is a polar molecule.

Wow! I learned so much from you. I will read more of your articles. Thank you!
Hey Horatio, glad to know that. Keep reading!
I love it
I am glad that you enjoyed the article. Keep Reading!